Reactions of peroxynitrite with uric acid: formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress
- PMID: 19219741
- PMCID: PMC2742358
- DOI: 10.1080/15257770902736400
Reactions of peroxynitrite with uric acid: formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress
Abstract
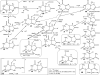
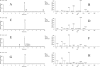
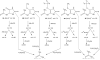
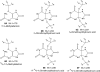
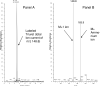
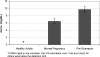
Hyperuricemia is associated with hypertension, metabolic syndrome, preeclampsia, cardio-vascular disease and renal disease, all conditions associated with oxidative stress. We hypothesized that uric acid, a known antioxidant, might become prooxidative following its reaction with oxidants; and, thereby contribute to the pathogenesis of these diseases. Uric acid and 1,3-(15)N(2)-uric acid were reacted with peroxynitrite in different buffers and in the presence of alcohols, antioxidants and in human plasma. The reaction products were identified using liquid chromatography-mass spectrometry (LC-MS) analyses. The reactions generate reactive intermediates that yielded triuret as their final product. We also found that the antioxidant, ascorbate, could partially prevent this reaction. Whereas triuret was preferentially generated by the reactions in aqueous buffers, when uric acid or 1,3-(15)N(2)-uric acid was reacted with peroxynitrite in the presence of alcohols, it yielded alkylated alcohols as the final product. By extension, this reaction can alkylate other biomolecules containing OH groups and others containing labile hydrogens. Triuret was also found to be elevated in the urine of subjects with preeclampsia, a pregnancy-specific hypertensive syndrome that is associated with oxidative stress, whereas very little triuret is produced in normal healthy volunteers. We conclude that under conditions of oxidative stress, uric acid can form reactive intermediates, including potential alkylating species, by reacting with peroxynitrite. These reactive intermediates could possibly explain how uric acid contributes to the pathogenesis of diseases such as the metabolic syndrome and hypertension.
Figures





References
-
- Wu XW, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J. Mo. Evo. 1992;34:78–84. - PubMed
-
- Kand'ar R, Zakova P, Muzakova V. Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clin. Chim. Acta. 2006;365:249–256. - PubMed
-
- Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: Implications for uncoupling endothelial nitric oxide synthase. Biochem. Pharmacol. 2005;70:343–354. - PubMed
-
- Robinson KM, Morre JT, Beckman JS. Triuret: a novel product of peroxynitrite-mediated oxidation of urate. Arch. Bioch. Biophys. 2004;423:213–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
