(S)-5-(p-nitrobenzyl)-PCTA, a promising bifunctional ligand with advantageous metal ion complexation kinetics
- PMID: 19220012
- PMCID: PMC2765549
- DOI: 10.1021/bc8004914
(S)-5-(p-nitrobenzyl)-PCTA, a promising bifunctional ligand with advantageous metal ion complexation kinetics
Abstract
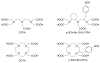
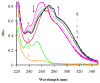
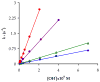
A bifunctional version of PCTA (3,6,9,15-tetraazabicyclo[9.3.1]pentadeca-1(15),11,13-triene-3,6,9,-triacetic acid) that exhibits fast complexation kinetics with the trivalent lanthanide(III) ions was synthesized in reasonable yields starting from N,N',N''-tristosyl-(S)-2-(p-nitrobenzyl)-diethylenetriamine. pH-potentiometric studies showed that the basicities of p-nitrobenzyl-PCTA and the parent ligand PCTA were similar. The stability of M(NO(2)-Bn-PCTA) (M = Mg(2+), Ca(2+), Cu(2+), Zn(2+)) complexes was similar to that of the corresponding PCTA complexes, while the stability of Ln(3+) complexes of the bifunctional ligand is somewhat lower than that of PCTA chelates. The rate of complex formation of Ln(NO(2)-Bn-PCTA) complexes was found to be quite similar to that of PCTA, a ligand known to exhibit the fastest formation rates among all lanthanide macrocyclic ligand complexes studied to date. The acid-catalyzed decomplexation kinetic studies of the selected Ln(NO(2)-Bn-PCTA) complexes showed that the kinetic inertness of the complexes was comparable to that of Ln(DOTA) chelates making the bifunctional ligand NO(2)-Bn-PCTA suitable for labeling biological vectors with radioisotopes for nuclear medicine applications.
Figures



Similar articles
-
Equilibrium and formation/dissociation kinetics of some Ln(III)PCTA complexes.Inorg Chem. 2006 Nov 13;45(23):9269-80. doi: 10.1021/ic0608750. Inorg Chem. 2006. PMID: 17083226 Free PMC article.
-
Synthesis, potentiometric, kinetic, and NMR Studies of 1,4,7,10-tetraazacyclododecane-1,7-bis(acetic acid)-4,10-bis(methylenephosphonic acid) (DO2A2P) and its complexes with Ca(II), Cu(II), Zn(II) and lanthanide(III) ions.Inorg Chem. 2008 May 5;47(9):3851-62. doi: 10.1021/ic7024704. Epub 2008 Apr 2. Inorg Chem. 2008. PMID: 18380456
-
Lanthanide(III) complexes of tris(amide) PCTA derivatives as potential bimodal magnetic resonance and optical imaging agents.Chemistry. 2009 Dec 7;15(47):13188-200. doi: 10.1002/chem.200901095. Chemistry. 2009. PMID: 19882595 Free PMC article.
-
Evaluation of novel bifunctional chelates for the development of Cu-64-based radiopharmaceuticals.Nucl Med Biol. 2008 Nov;35(8):875-82. doi: 10.1016/j.nucmedbio.2008.09.001. Nucl Med Biol. 2008. PMID: 19026949
-
64Cu-Labeled Oxo-DO3A-conjugated trastuzumab and PCTA-conjugated trastuzumab.2012 Jul 24 [updated 2012 Aug 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Jul 24 [updated 2012 Aug 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22934320 Free Books & Documents. Review.
Cited by
-
Pycup--a bifunctional, cage-like ligand for (64)Cu radiolabeling.Mol Pharm. 2014 Feb 3;11(2):617-29. doi: 10.1021/mp400686z. Epub 2013 Dec 11. Mol Pharm. 2014. PMID: 24294970 Free PMC article.
-
Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers.Chem Rev. 2019 Jan 23;119(2):957-1057. doi: 10.1021/acs.chemrev.8b00363. Epub 2018 Oct 16. Chem Rev. 2019. PMID: 30350585 Free PMC article. Review.
-
Synthesis of 12-Membered Tetra-aza Macrocyclic Pyridinophanes Bearing Electron-Withdrawing Groups.J Org Chem. 2020 Apr 3;85(7):4988-4998. doi: 10.1021/acs.joc.0c00188. Epub 2020 Mar 25. J Org Chem. 2020. PMID: 32208700 Free PMC article.
-
Versatile Macrocyclic Platform for the Complexation of [natY/90Y]Yttrium and Lanthanide Ions.Inorg Chem. 2022 Apr 25;61(16):6209-6222. doi: 10.1021/acs.inorgchem.2c00378. Epub 2022 Apr 14. Inorg Chem. 2022. PMID: 35418232 Free PMC article.
-
Dialing in on pharmacological features for a therapeutic antioxidant small molecule.Dalton Trans. 2019 Sep 7;48(33):12430-12439. doi: 10.1039/c9dt01800j. Epub 2019 Jul 25. Dalton Trans. 2019. PMID: 31342985 Free PMC article.
References
-
- De Leon-Rodriguez LM, Kovacs Z. The synthesis and chelation chemistry of DOTA-peptide conjugates. Bioconjugate Chem. 2008;19:391–402. - PubMed
-
- Breeman WAP, de Blois E, Bakker WH, Krenning EP. Radionuclide peptide cancer therapy. In: Chinol M, Paganelli G, editors. Radionuclide Peptide Cancer Therapy. Taylor & Francis; New York: 2006. pp. 119–125.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

