Antiparkinsonian drug-induced sleepiness: a double-blind placebo-controlled study of L-dopa, bromocriptine and pramipexole in healthy subjects
- PMID: 19220275
- PMCID: PMC2675044
- DOI: 10.1111/j.1365-2125.2008.03310.x
Antiparkinsonian drug-induced sleepiness: a double-blind placebo-controlled study of L-dopa, bromocriptine and pramipexole in healthy subjects
Abstract
Aims: To assess the sleepiness induced by pramipexole, a D2/D3-dopamine receptor agonist commonly used in Parkinson's disease and restless legs syndrome, without the problem of the confounding factors related to the disease.
Methods: Placebo, bromocriptine (2.5 mg), L-dopa (100 mg) and pramipexole (0.5 mg) were administered in a single oral dose on four separate days, with at least a 2-week wash-out period in a randomized cross-over design. Induced somnolence was assessed using Multiple Sleep Latency Test (MSLT) and subjective scaling of vigilance. Twelve male subjects (26.3 +/- 5.5 years old) without anxiety, mood, sleep or sedation disorders were enrolled.
Results: Pramipexole significantly reduced mean sleep latency compared with placebo 3 h 30 min [-6.1 min (-9.8, -2.4), P = 0.002] and 5 h 30 min [-5.6 min (-7.7, -3.5), P = 0.003] after administration. In addition, the total duration of sleep during the tests was higher with pramipexole than with placebo [+6.0 min (2.3, 9.7), P < 0.001]. These differences were not observed with L-dopa and bromocriptine in comparison with placebo. The induced sleepiness was not associated with an increase in subjective somnolence scaling, indicating that this adverse event may occur without prior warning.
Conclusions: These results show that a single oral dose of pramipexole induces sleepiness as assessed by MSLT in healthy young subjects, independent of disease-related sleep dysfunction.
Figures
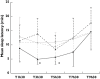
 ); Placebo (
); Placebo ( ); Bromocriptine (
); Bromocriptine ( ); Pramipexole (
); Pramipexole ( )
)
 ); Placebo (
); Placebo ( ); Bromocriptine (
); Bromocriptine ( ); Pramipexole (
); Pramipexole ( )
)References
-
- Arnulf I. Excessive daytime sleepiness in parkinsonism. Sleep Med Rev. 2005;9:185–200. - PubMed
-
- Frucht S, Rogers JD, Greene PE, Gordon MF, Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology. 1999;52:1908–10. - PubMed
-
- Hauser RA, Gauger L, Anderson WM, Zesiewicz TA. Pramipexole-induced somnolence and episodes of daytime sleep. Mov Disord. 2000;15:658–63. - PubMed
-
- Paladini D. Sleep attacks in two parkinson's disease patients taking ropinirole. Mov Disord. 2000;15:130–1.
-
- Ferreira JJ, Galitzky M, Montastruc JL, Rascol O. Sleep attacks and Parkinson's disease treatment. Lancet. 2000;355:1333–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

