Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster
- PMID: 19220479
- PMCID: PMC4014202
- DOI: 10.1111/j.1601-183X.2009.00483.x
Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster
Abstract
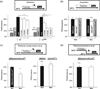
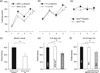
Drosophila melanogaster is increasingly being used to model human conditions that are associated with cognitive deficits including fragile-X syndrome, Alzheimer's disease, Parkinson's disease, sleep loss, etc. With few exceptions, cognitive abilities that are known to be modified in these conditions in humans have not been evaluated in fly models. One reason is the absence of a simple, inexpensive and reliable behavioral assay that can be used by laboratories that are not expert in learning and memory. Aversive phototaxic suppression (APS) is a simple assay in which flies learn to avoid light that is paired with an aversive stimulus (quinine/humidity). However, questions remain about whether the change in the fly's behavior reflects learning an association between light and quinine/humidity or whether the change in behavior is because of nonassociative effects of habituation and/or sensitization. We evaluated potential effects of sensitization and habituation on behavior in the T-maze and conducted a series of yoked control experiments to further exclude nonassociative effects and determine whether this task evaluates operant learning. Together these experiments indicate that a fly must associate the light with quinine/humidity to successfully complete the task. Next, we show that five classic memory mutants are deficient in this assay. Finally, we evaluate performance in a fly model of neurodegenerative disorders associated with the accumulation of Tau. These data indicate that APS is a simple and effective assay that can be used to evaluate fly models of human conditions associated with cognitive deficits.
Figures



References
-
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. - PubMed
-
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, Broger C, Tully T. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

