Novel mechanism of U18666A-induced tumour necrosis factor-alpha production in RAW 264.7 macrophage cells
- PMID: 19220841
- PMCID: PMC2669532
- DOI: 10.1111/j.1365-2249.2008.03779.x
Novel mechanism of U18666A-induced tumour necrosis factor-alpha production in RAW 264.7 macrophage cells
Abstract
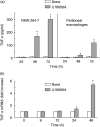
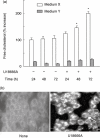
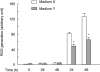
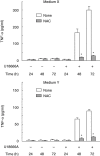
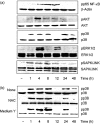
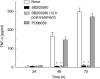
U18666A is a cholesterol transport-inhibiting agent that is used widely to mimic Niemann-Pick type C disease. The effect of U18666A on tumour necrosis factor (TNF)-alpha production in mouse macrophage cell line, RAW 264.7 cells and peritoneal macrophages was examined. U18666A induced TNF-alpha mRNA expression 48 h after the treatment, and TNF-alpha production 48 and 72 h after stimulation in RAW 264.7 cells. U18666A accumulated intracellular free cholesterol in the culture of normal medium but not cholesterol-free medium. U18666A also induced reactive oxygen species (ROS) generation in normal medium but much less in cholesterol-free medium. Anti-oxidant N-acetyl-L-cysteine (NAC) abolished U18666A-induced TNF-alpha production. U18666A led to the phosphorylation of p38 mitogen-activated protein kinase 24 and 48 h after the stimulation and the p38 activation was inhibited in presence of cholesterol-free medium or NAC. A p38 inhibitor reduced U18666A-induced TNF-alpha production. Taken together, U18666A was suggested to induce TNF-alpha production in RAW 264.7 cells via free cholesterol accumulation-mediated ROS generation.
Figures
Similar articles
-
Hydrogen peroxide induces the production of tumor necrosis factor-alpha in RAW 264.7 macrophage cells via activation of p38 and stress-activated protein kinase.Innate Immun. 2008 Jun;14(3):190-6. doi: 10.1177/1753425908093932. Innate Immun. 2008. PMID: 18562577
-
Chronic exposure to U18666A induces apoptosis in cultured murine cortical neurons.Biochem Biophys Res Commun. 2004 Mar 5;315(2):408-17. doi: 10.1016/j.bbrc.2004.01.066. Biochem Biophys Res Commun. 2004. PMID: 14766223
-
U18666A Treatment Results in Cholesterol Accumulation, Reduced Na(+), K(+)-ATPase Activity, and Increased Oxidative Stress in Rat Cortical Astrocytes.Lipids. 2015 Oct;50(10):937-44. doi: 10.1007/s11745-015-4062-4. Epub 2015 Sep 7. Lipids. 2015. PMID: 26344921
-
Cellular mechanism of U18666A-mediated apoptosis in cultured murine cortical neurons: bridging Niemann-Pick disease type C and Alzheimer's disease.Cell Signal. 2006 Nov;18(11):1844-53. doi: 10.1016/j.cellsig.2006.04.006. Epub 2006 May 7. Cell Signal. 2006. PMID: 16797161 Review.
-
Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes.Lipids. 2009 Jun;44(6):477-87. doi: 10.1007/s11745-009-3305-7. Epub 2009 May 14. Lipids. 2009. PMID: 19440746 Review.
Cited by
-
Disruption in connexin-based communication is associated with intracellular Ca²⁺ signal alterations in astrocytes from Niemann-Pick type C mice.PLoS One. 2013 Aug 15;8(8):e71361. doi: 10.1371/journal.pone.0071361. eCollection 2013. PLoS One. 2013. PMID: 23977027 Free PMC article.
-
Seladin-1 is a novel lipopolysaccharide (LPS)-responsive gene and inhibits the tumour necrosis factor-alpha production and osteoclast formation in response to LPS.Immunology. 2010 Sep;131(1):59-66. doi: 10.1111/j.1365-2567.2010.03274.x. Epub 2010 Apr 6. Immunology. 2010. PMID: 20406300 Free PMC article.
-
Anti-TNF therapy for inflammatory bowel disease in patients with neurodegenerative Niemann-Pick disease Type C.Wellcome Open Res. 2022 Jan 11;7:11. doi: 10.12688/wellcomeopenres.16986.1. eCollection 2022. Wellcome Open Res. 2022. PMID: 35694196 Free PMC article.
-
The expression of neuronal sorting nexin 8 (SNX8) exacerbates abnormal cholesterol levels.J Mol Neurosci. 2014 May;53(1):125-34. doi: 10.1007/s12031-013-0209-z. Epub 2013 Dec 21. J Mol Neurosci. 2014. PMID: 24362679
References
-
- Mohammadi A, Perry RJ, Storey MK, et al. Golgi localization and phosphorylation of oxysterol binding protein in Niemann–Pick C and U18666a-treated cells. J Lipid Res. 2001;42:1062–71. - PubMed
-
- Liscum L, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-β-[2-(diethylamino)ethoxy]androst-5-en-17-one. J Biol Chem. 1989;264:11796–06. - PubMed
-
- Hall AM, Krishnamoorthy L, Orlow SJ. Accumulation of tyrosinase in the endolysosomal compartment is induced by U18666A. Pigment Cell Res. 2003;16:149–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

