A randomised trial of conventional versus BAUS procedure-specific consent forms for transurethral resection of prostate
- PMID: 19220941
- PMCID: PMC2765012
- DOI: 10.1308/003588409X359277
A randomised trial of conventional versus BAUS procedure-specific consent forms for transurethral resection of prostate
Abstract
Introduction: Conventional consent forms often contain incomplete information regarding risks associated with invasive procedures. BAUS has introduced procedure-specific consent forms (PSCF) documenting the risks associated with urological procedures. We compared patients' understanding of the risks and benefits of TURP after the consenting process with either conventional documentation or PSCF.
Patients and methods: One hundred patients were randomised to be consented with either a conventional or PSCF. After 3 h, their understanding was assessed with a questionnaire asking patients to document the indication and likelihood of symptomatic improvement, estimate frequency of complications and the risk of future re-operation. Data were compared by Mann-Whitney test.
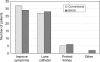
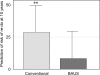
Results: Fifty patients were randomised to each group. There was no significant difference in mean age, grade of doctor obtaining consent or time interval from consent to questionnaire. Both groups accurately predicted the chance of improved symptoms (median, 80%). There was no significant difference in patients' median estimation of risk of complications such as incontinence, erectile dysfunction, or retrograde ejaculation. Patients consented with the PSCF predicted the risk of re-operation more accurately (median answer, 10% versus 30%; P = 0.007, Mann-Whitney test).
Conclusions: Recall of data was sub-optimal in both groups. For most data points there was no significant difference in estimation of risks between groups. Those consented with a procedure-specific consent form predicted risk of re-operation at 10 years more accurately. Procedure-specific consent forms offer an advantage over conventional consent in this study. We feel that the provision of a written structured framework allows better informed consent for TURP.
Figures

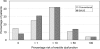
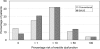

References
-
- General Medical Council. The Ethical Considerations. London: GMC; 1998. Seeking Patients' Consent.
-
- Department of Health. Making amends; a consultation paper setting out the proposals for reforming the approach to clinical negligence in the NHS. London: DH; 2003.
-
- Department of Health. NHSLA report and accounts. London: DH; 2005.
-
- Chester v Afshar. In UKHL. 2004; 41.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

