Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells
- PMID: 19221016
- PMCID: PMC2670666
- DOI: 10.1152/ajpgi.90519.2008
Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells
Abstract
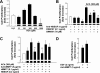
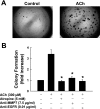
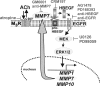
Previously, we showed that ACh-induced proliferation of human colon cancer cells is mediated by transactivation of epidermal growth factor (EGF) receptors (EGFRs). In the present study, we elucidate the molecular mechanism underlying this action. ACh-induced proliferation of H508 colon cancer cells, which express exclusively M3 muscarinic receptors (M3Rs), was attenuated by anti-EGFR ligand binding domain antibody, a broad-spectrum matrix metalloproteinase (MMP) inhibitor, anti-MMP7 antibody, a diphtheria toxin analog that blocks release of an EGFR ligand [heparin-binding EGF-like growth factor (HBEGF)], and anti-HBEGF antibody. Conditioned media from ACh-treated H508 cells induced proliferation of SNU-C4 colon cancer cells that express EGFR but not M3R. These actions were attenuated by an EGFR inhibitor and by anti-EGFR and anti-HBEGF antibodies. In H508, but not SNU-C4, colon cancer cells, ACh caused a striking dose- and time-dependent increase in levels of MMP7 mRNA and MMP7 protein. Similarly, ACh induced robust MMP1 and MMP10 gene transcription. ACh-induced MMP1, MMP7, and MMP10 gene transcription was attenuated by atropine, anti-EGFR antibody, and chemical inhibitors of EGFR and ERK activation. In contrast, inhibitors of phosphatidylinositol 3-kinase and NF-kappaB activation did not alter MMP gene transcription. Collectively, these findings indicate that MMP7-catalyzed release of HBEGF mediates ACh-induced transactivation of EGFR and consequent proliferation of colon cancer cells. ACh-induced activation of EGFR and downstream ERK signaling also regulates transcriptional activation of MMP7, thereby identifying a novel feed-forward mechanism for neoplastic cell proliferation.
Figures




Similar articles
-
Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion.Am J Physiol Gastrointest Liver Physiol. 2011 May;300(5):G749-60. doi: 10.1152/ajpgi.00306.2010. Epub 2011 Jan 27. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21273532 Free PMC article.
-
Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line.Biochem Pharmacol. 2007 Apr 1;73(7):1001-12. doi: 10.1016/j.bcp.2006.11.028. Epub 2006 Dec 10. Biochem Pharmacol. 2007. PMID: 17222808 Free PMC article.
-
Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells.Cancer Res. 2003 Oct 15;63(20):6744-50. Cancer Res. 2003. PMID: 14583469
-
Targeting M3 Muscarinic Receptors for Colon Cancer Therapy.Curr Mol Pharmacol. 2018;11(3):184-190. doi: 10.2174/1874467211666180119115828. Curr Mol Pharmacol. 2018. PMID: 29357811 Free PMC article. Review.
-
Overcoming Obstacles to Targeting Muscarinic Receptor Signaling in Colorectal Cancer.Int J Mol Sci. 2021 Jan 13;22(2):716. doi: 10.3390/ijms22020716. Int J Mol Sci. 2021. PMID: 33450835 Free PMC article. Review.
Cited by
-
Matrix metalloproteinases as biomarkers and therapeutic targets in colitis-associated cancer.Front Oncol. 2024 Jan 15;13:1325095. doi: 10.3389/fonc.2023.1325095. eCollection 2023. Front Oncol. 2024. PMID: 38288108 Free PMC article. Review.
-
Bile acid: a potential inducer of colon cancer stem cells.Stem Cell Res Ther. 2016 Dec 1;7(1):181. doi: 10.1186/s13287-016-0439-4. Stem Cell Res Ther. 2016. PMID: 27908290 Free PMC article.
-
Slc10a2-null mice uncover colon cancer-promoting actions of endogenous fecal bile acids.Carcinogenesis. 2015 Oct;36(10):1193-200. doi: 10.1093/carcin/bgv107. Epub 2015 Jul 25. Carcinogenesis. 2015. PMID: 26210740 Free PMC article.
-
Nerve-cancer interactions in the stromal biology of pancreatic cancer.Front Physiol. 2012 Apr 17;3:97. doi: 10.3389/fphys.2012.00097. eCollection 2012. Front Physiol. 2012. PMID: 22529816 Free PMC article.
-
Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion.Am J Physiol Gastrointest Liver Physiol. 2011 May;300(5):G749-60. doi: 10.1152/ajpgi.00306.2010. Epub 2011 Jan 27. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21273532 Free PMC article.
References
-
- Adachi Y, Yamamoto H, Itoh F, Arimura Y, Nishi M, Endo T, Imai K. Clinicopathologic and prognostic significance of matrilysin expression at the invasive front in human colorectal cancers. Int J Cancer 95: 290–294, 2001. - PubMed
-
- Brinckerhoff CE, Plucinska IM, Sheldon LA, O'Connor GT. Half-life of synovial cell collagenase mRNA is modulated by phorbol myristate acetate but not by all-trans-retinoic acid or dexamethasone. Biochemistry 25: 6378–6384, 1986. - PubMed
-
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253: 269–285, 2003. - PubMed
-
- Cheng K, Chen Y, Zimniak P, Raufman J, Xiao Y, Frucht H. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim Biophys Acta 1588: 48–55, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

