A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry
- PMID: 19221033
- PMCID: PMC2651283
- DOI: 10.1073/pnas.0813346106
A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry
Abstract
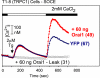
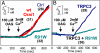
TRPC and Orai proteins have both been proposed to form Ca(2+)-selective, store-operated calcium entry (SOCE) channels that are activated by store-depletion with Ca(2+) chelators or calcium pump inhibitors. In contrast, only TRPC proteins have been proposed to form nonselective receptor-operated calcium entry (ROCE) cation channels that are activated by Gq/Gi-PLCbeta signaling, which is the physiological stimulus for store depletion. We reported previously that a dominant negative Orai1 mutant, R91W, inhibits Ca(2+) entry through both SOCE and ROCE channels, implicating Orai participation in both channel complexes. However, the argument for Orai participating in ROCE independently of store depletion is tenuous because store depletion is an integral component of the ROCE response, which includes formation of IP3, a store-depleting agent. Here we show that the R91W mutant also blocks diacylglycerol (DAG)-activated Ca(2+) entry into cells that stably, or transiently, express DAG-responsive TRPC proteins. This strongly suggests that Orai and TRPC proteins form complexes that participate in Ca(2+) entry with or without activation of store depletion. To integrate these results with recent data linking SOCE with recruitment of Orai and TRPCs to lipid rafts by STIM, we develop the hypothesis that Orai:TRPC complexes recruited to lipid rafts mediate SOCE, whereas the same complexes mediate ROCE when they are outside of lipid rafts. It remains to be determined whether the molecules forming the permeation pathway are the same when Orai:TRPC complexes mediate ROCE or SOCE.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

