Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves
- PMID: 19221394
- PMCID: PMC2699134
- DOI: 10.1084/jem.20082129
Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves
Abstract
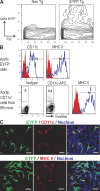
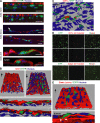
Presumptive dendritic cells (DCs) bearing the CD11c integrin and other markers have previously been identified in normal mouse and human aorta. We used CD11c promoter-enhanced yellow fluorescent protein (EYFP) transgenic mice to visualize aortic DCs and study their antigen-presenting capacity. Stellate EYFP(+) cells were readily identified in the aorta and could be double labeled with antibodies to CD11c and antigen-presenting major histocompatability complex (MHC) II products. The DCs proved to be particularly abundant in the cardiac valves and aortic sinus. In all aortic locations, the CD11c(+) cells localized to the subintimal space with occasional processes probing the vascular lumen. Aortic DCs expressed little CD40 but expressed low levels of CD1d, CD80, and CD86. In studies of antigen presentation, DCs selected on the basis of EYFP expression or binding of anti-CD11c antibody were as effective as DCs similarly selected from the spleen. In particular, the aortic DCs could cross-present two different protein antigens on MHC class I to CD8(+) TCR transgenic T cells. In addition, after intravenous injection, aortic DCs could capture anti-CD11c antibody and cross-present ovalbumin to T cells. These results indicate that bona fide DCs are a constituent of the normal aorta and cardiac valves.
Figures



References
-
- Ma-Krupa W., Kwan M., Goronzy J.J., Weyand C.M. 2005. Toll-like receptors in giant cell arteritis.Clin. Immunol. 115:38–46 - PubMed
-
- Shimizu K., Mitchell R.N., Libby P. 2006. Inflammation and cellular immune responses in abdominal aortic aneurysms.Arterioscler. Thromb. Vasc. Biol. 26:987–994 - PubMed
-
- Hansson G.K., Libby P. 2006. The immune response in atherosclerosis: a double-edged sword.Nat. Rev. Immunol. 6:508–519 - PubMed
-
- Steinman R.M., Hemmi H. 2006. Dendritic cells: translating innate to adaptive immunity.Curr. Top. Microbiol. Immunol. 311:17–58 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

