Abeta-dependent Inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE
- PMID: 19221410
- PMCID: PMC3726279
- DOI: 10.3233/JAD-2009-1045
Abeta-dependent Inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE
Abstract
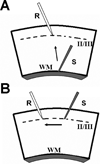
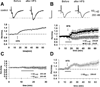
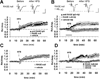
Oligomeric amyloid-beta (Abeta) interferes with long-term potentiation (LTP) and cognitive processes, suggesting that Abeta peptides may play a role in the neuronal dysfunction which characterizes the early stages of Alzheimer's disease (AD). Multiple lines of evidence have highlighted RAGE (receptor for advanced glycation end-products) as a receptor involved in Abeta-induced neuronal and synaptic dysfunction. In the present study, we investigated the effect of oligomeric soluble Abeta1-42 on LTP elicited by the stimulation of different intracortical pathways in the mouse visual cortex. A variety of nanomolar concentrations (20-200 nM) of Abeta1-42 were able to inhibit LTP in cortical layer II-III induced by either white matter (WM-Layer II/III) or the layer II/III (horizontal pathway) stimulation, whereas the inhibition of LTP was more susceptible to Abeta1-42, which occurred at 20 nM of Abeta, when stimulating layer II-III horizontal pathway. Remarkably, cortical slices were resistant to nanomolar Abeta1-42 in the absence of RAGE (genetic deletion of RAGE) or blocking RAGE by RAGE antibody. These results indicate that nanomolar Abeta inhibits LTP expression in different neocortical circuits. Crucially, it is demonstrated that Abeta-induced reduction of LTP in different cortical pathways is mediated by RAGE.
Figures


References
-
- Stromer T, Serpell LC. Structure and morphology of the Alzheimer’s amyloid fibril. Microsc Res Tech. 2005;67:210–217. - PubMed
-
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo . Nature. 2002;416:535–539. - PubMed
-
- Wang QW, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004;24:3370–3378. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

