Cryptic peptides of the kringle domains preferentially bind to disease-associated prion protein
- PMID: 19221431
- PMCID: PMC2741132
- DOI: 10.3233/JAD-2009-0980
Cryptic peptides of the kringle domains preferentially bind to disease-associated prion protein
Abstract
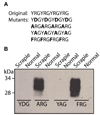
Prion diseases are a group of fatal neurodegenerative disorders characterized by the accumulation of a misfolded form (PrP(Sc)) of the cellular prion protein (PrP(C)) in the brains of affected individuals. The conversion of PrP(C) to PrP(Sc) is thought to involve a change in protein conformation from a normal, primarily alpha-helical structure into a beta-sheet conformer. Few proteins have been identified that differentially interact with the two forms of PrP. It has been reported that plasminogen binds to PrP(Sc) from a variety of prion phenotypes. We have examined potential motifs within the kringle region that may be responsible for binding to PrP. We synthesized 12-15-mer peptides that contain small, repetitive stretches of amino acid residues found within the kringle domains of plasminogen. These synthetic peptides were found to capture PrP(Sc) from the brain homogenates of bovine spongiform encephalopathy affected cattle, chronic wasting disease affected elk, experimental scrapie of hamsters and that of subjects affected by Creutzfeldt-Jakob disease, without binding to PrP(C) in unaffected controls. Therefore, we have identified critical peptide motifs that may be important for protein-protein interactions in prion disease pathogenesis. The ability of these synthetic peptides to bind preferentially to PrP(Sc) suggests a potential application in the diagnosis of prion diseases.
Figures

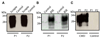





References
-
- Gambetti P, et al. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–239. - PubMed
-
- Will RG, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347(9006):921–925. - PubMed
-
- Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood. 1991;78(12):3114–3124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

