Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice
- PMID: 19221439
- PMCID: PMC2648694
- DOI: 10.1172/JCI35541
Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice
Abstract
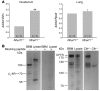
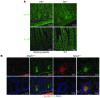
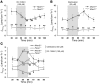
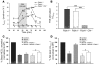
The epithelial anion channel CFTR interacts with multiple PDZ domain-containing proteins. Heterologous expression studies have demonstrated that the Na+/H+ exchanger regulatory factors, NHERF1, NHERF2, and PDZK1 (NHERF3), modulate CFTR membrane retention, conductivity, and interactions with other transporters. To study their biological roles in vivo, we investigated CFTR-dependent duodenal HCO3- secretion in mouse models of Nherf1, Nherf2, and Pdzk1 loss of function. We found that Nherf1 ablation strongly reduced basal as well as forskolin-stimulated (FSK-stimulated) HCO3- secretory rates and blocked beta2-adrenergic receptor (beta2-AR) stimulation. Conversely, Nherf2-/- mice displayed augmented FSK-stimulated HCO3- secretion. Furthermore, although lysophosphatidic acid (LPA) inhibited FSK-stimulated HCO3- secretion in WT mice, this effect was lost in Nherf2-/- mice. Pdzk1 ablation reduced basal, but not FSK-stimulated, HCO3- secretion. In addition, laser microdissection and quantitative PCR revealed that the beta2-AR and the type 2 LPA receptor were expressed together with CFTR in duodenal crypts and that colocalization of the beta2-AR and CFTR was reduced in the Nherf1-/- mice. These data suggest that the NHERF proteins differentially modulate duodenal HCO3- secretion: while NHERF1 is an obligatory linker for beta2-AR stimulation of CFTR, NHERF2 confers inhibitory signals by coupling the LPA receptor to CFTR.
Figures
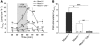


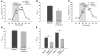
Similar articles
-
Down regulation of small intestinal ion transport in PDZK1- (CAP70/NHERF3) deficient mice.Pflugers Arch. 2007 Jul;454(4):575-86. doi: 10.1007/s00424-007-0239-x. Epub 2007 Mar 9. Pflugers Arch. 2007. PMID: 17347851
-
Both NHERF3 and NHERF2 are necessary for multiple aspects of acute regulation of NHE3 by elevated Ca2+, cGMP, and lysophosphatidic acid.Am J Physiol Gastrointest Liver Physiol. 2018 Jan 1;314(1):G81-G90. doi: 10.1152/ajpgi.00140.2017. Epub 2017 Sep 7. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 28882822 Free PMC article.
-
Knockout mouse models for intestinal electrolyte transporters and regulatory PDZ adaptors: new insights into cystic fibrosis, secretory diarrhoea and fructose-induced hypertension.Exp Physiol. 2009 Feb;94(2):175-9. doi: 10.1113/expphysiol.2008.043018. Epub 2008 Oct 17. Exp Physiol. 2009. PMID: 18931049 Review.
-
Na+/H+ exchanger regulatory factor isoform 1 overexpression modulates cystic fibrosis transmembrane conductance regulator (CFTR) expression and activity in human airway 16HBE14o- cells and rescues DeltaF508 CFTR functional expression in cystic fibrosis cells.J Biol Chem. 2005 Dec 9;280(49):40925-33. doi: 10.1074/jbc.M505103200. Epub 2005 Oct 3. J Biol Chem. 2005. PMID: 16203733
-
CFTR-NHERF2-LPA₂ Complex in the Airway and Gut Epithelia.Int J Mol Sci. 2017 Sep 4;18(9):1896. doi: 10.3390/ijms18091896. Int J Mol Sci. 2017. PMID: 28869532 Free PMC article. Review.
Cited by
-
Integrating the puzzle pieces: the current atomistic picture of phospholipid-G protein coupled receptor interactions.Biochim Biophys Acta. 2013 Jan;1831(1):2-12. doi: 10.1016/j.bbalip.2012.09.002. Epub 2012 Sep 12. Biochim Biophys Acta. 2013. PMID: 22982815 Free PMC article. Review.
-
Lysophosphatidic acid 5 receptor induces activation of Na(+)/H(+) exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells.Am J Physiol Cell Physiol. 2011 Nov;301(5):C1008-16. doi: 10.1152/ajpcell.00231.2011. Epub 2011 Aug 10. Am J Physiol Cell Physiol. 2011. PMID: 21832242 Free PMC article.
-
Expression of lysophosphatidic acid receptor 5 is necessary for the regulation of intestinal Na+/H+ exchanger 3 by lysophosphatidic acid in vivo.Am J Physiol Gastrointest Liver Physiol. 2018 Oct 1;315(4):G433-G442. doi: 10.1152/ajpgi.00130.2018. Epub 2018 May 24. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29792531 Free PMC article.
-
The electroneutral Na⁺:HCO₃⁻ cotransporter NBCn1 is a major pHi regulator in murine duodenum.J Physiol. 2012 Jul 15;590(14):3317-33. doi: 10.1113/jphysiol.2011.226506. Epub 2012 May 14. J Physiol. 2012. PMID: 22586225 Free PMC article.
-
Molecular transport machinery involved in orchestrating luminal acid-induced duodenal bicarbonate secretion in vivo.J Physiol. 2013 Nov 1;591(21):5377-91. doi: 10.1113/jphysiol.2013.254854. Epub 2013 Sep 9. J Physiol. 2013. PMID: 24018950 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

