Short-term starvation of immune deficient Drosophila improves survival to gram-negative bacterial infections
- PMID: 19221590
- PMCID: PMC2637427
- DOI: 10.1371/journal.pone.0004490
Short-term starvation of immune deficient Drosophila improves survival to gram-negative bacterial infections
Abstract
Background: Primary immunodeficiencies are inborn errors of immunity that lead to life threatening conditions. These predispositions describe human immunity in natura and highlight the important function of components of the Toll-IL-1- receptor-nuclear factor kappa B (TIR-NF-kappaB) pathway. Since the TIR-NF-kappaB circuit is a conserved component of the host defence in higher animals, genetically tractable models may contribute ideas for clinical interventions.
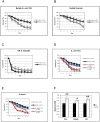
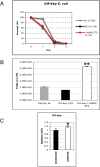
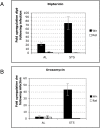
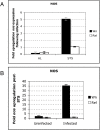
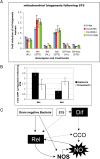
Methodology/principal findings: We used immunodeficient fruit flies (Drosophila melanogaster) to address questions pertaining to survival following bacterial infection. We describe here that flies lacking the NF-kappaB protein Relish, indispensable for countering Gram-negative bacteria, had a greatly improved survival to such infections when subject to dietary short-term starvation (STS) prior to immune challenge. STS induced the release of Nitric Oxide (NO), a potent molecule against pathogens in flies, mice and humans. Administering the NO Synthase-inhibitory arginine analog N-Nitro-L-Arginine-Methyl-Ester (L-NAME) but not its inactive enantiomer D-NAME increased once again sensitivity to infection to levels expected for relish mutants. Surprisingly, NO signalling required the NF-kappaB protein Dif, usually needed for responses against Gram-positive bacteria.
Conclusions/significance: Our results show that NO release through STS may reflect an evolutionary conserved process. Moreover, STS could be explored to address immune phenotypes related to infection and may offer ways to boost natural immunity.
Conflict of interest statement
Figures

References
-
- Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317:617–619. - PubMed
-
- Picard C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. - PubMed
-
- Zhang, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition in innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Wang L, Ligoxygakis P. Pathogen sensing and signalling in Drosophila immune response. Immunobiol. 2006;211:251–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

