Is paromomycin an effective and safe treatment against cutaneous leishmaniasis? A meta-analysis of 14 randomized controlled trials
- PMID: 19221595
- PMCID: PMC2637543
- DOI: 10.1371/journal.pntd.0000381
Is paromomycin an effective and safe treatment against cutaneous leishmaniasis? A meta-analysis of 14 randomized controlled trials
Abstract
Background: High cost, poor compliance, and systemic toxicity have limited the use of pentavalent antimony compounds (SbV), the treatment of choice for cutaneous leishmaniasis (CL). Paromomycin (PR) has been developed as an alternative to SbV, but existing data are conflicting.
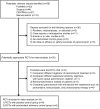
Methodology/principal findings: We searched PubMed, Scopus, and Cochrane Central Register of Controlled Trials, without language restriction, through August 2007, to identify randomized controlled trials that compared the efficacy or safety between PR and placebo or SbV. Primary outcome was clinical cure, defined as complete healing, disappearance, or reepithelialization of all lesions. Data were extracted independently by two investigators, and pooled using a random-effects model. Fourteen trials including 1,221 patients were included. In placebo-controlled trials, topical PR appeared to have therapeutic activity against the old world and new world CL, with increased local reactions, when used with methylbenzethonium chloride (MBCL) compared to when used alone (risk ratio [RR] for clinical cure, 2.58 versus 1.01: RR for local reactions, 1.60 versus 1.07). In SbV-controlled trials, the efficacy of topical PR was not significantly different from that of intralesional SbV in the old world CL (RR, 0.70; 95% confidence interval, 0.26-1.89), whereas topical PR was inferior to parenteral SbV in treating the new world CL (0.67; 0.54-0.82). No significant difference in efficacy was found between parenteral PR and parenteral SbV in the new world CL (0.88; 0.56-1.38). Systemic side effects were fewer with topical or parenteral PR than parenteral SbV.
Conclusions/significance: Topical PR with MBCL could be a therapeutic alternative to SbV in selected cases of the old world CL. Development of new formulations with better efficacy and tolerability remains to be an area of future research.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- World Health Organization. Leishmaniasis. 2007. Available: http://www.who.int/leishmaniasis/en via Internet. Accessed 17 Jun 2007.
-
- Buffet PA, Morizot G. Cutaneous leishmaniasis in France: towards the end of injectable therapy? Bull Soc Pathol Exot. 2003;96:383–8. - PubMed
-
- Armijos RX, Weigel MM, Calvopina M, Mancheno M, Rodriguez R. Comparison of the effectiveness of two topical paromomycin treatments versus meglumine antimoniate for New World cutaneous leishmaniasis. Acta Trop. 2004;91:153–160. - PubMed
-
- Correia D, Macedo VO, Carvalho EM, Barral A, Magalhaes AV, et al. Comparative study between meglumine antimoniato, pentamidine isothianate and aminosidine sulphate, in the treatment cutaneous primary lesions, caused by Leishmania (Viannia) braziliensis (L(V)b). Rev Soc Bras Med Trop. 1996;29:447–453. - PubMed
-
- Hepburn NC, Tidman MJ, Hunter JA. Aminosidine (paromomycin) versus sodium stibogluconate for the treatment of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1994;88:700–703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

