High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli
- PMID: 19221601
- PMCID: PMC2637983
- DOI: 10.1371/journal.pone.0004489
High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli
Abstract
Background: Artemisinin derivatives are the key active ingredients in Artemisinin combination therapies (ACTs), the most effective therapies available for treatment of malaria. Because the raw material is extracted from plants with long growing seasons, artemisinin is often in short supply, and fermentation would be an attractive alternative production method to supplement the plant source. Previous work showed that high levels of amorpha-4,11-diene, an artemisinin precursor, can be made in Escherichia coli using a heterologous mevalonate pathway derived from yeast (Saccharomyces cerevisiae), though the reconstructed mevalonate pathway was limited at a particular enzymatic step.
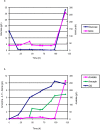
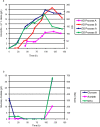
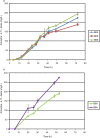
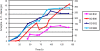
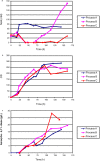
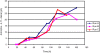
Methodology/ principal findings: By combining improvements in the heterologous mevalonate pathway with a superior fermentation process, commercially relevant titers were achieved in fed-batch fermentations. Yeast genes for HMG-CoA synthase and HMG-CoA reductase (the second and third enzymes in the pathway) were replaced with equivalent genes from Staphylococcus aureus, more than doubling production. Amorpha-4,11-diene titers were further increased by optimizing nitrogen delivery in the fermentation process. Successful cultivation of the improved strain under carbon and nitrogen restriction consistently yielded 90 g/L dry cell weight and an average titer of 27.4 g/L amorpha-4,11-diene.
Conclusions/ significance: Production of >25 g/L amorpha-4,11-diene by fermentation followed by chemical conversion to artemisinin may allow for development of a process to provide an alternative source of artemisinin to be incorporated into ACTs.
Conflict of interest statement
Figures

References
-
- Korenromp EMJ, Nahlen B, Wardlaw T, Young M. World Malaria Report 2005. Geneva: Roll Back Malaria; World Health Organization; UNICEF; 2005.
-
- Bloland P. Drug resistance in malaria. Geneva: World Health Organization; 2001.
-
- Olumese P. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2006.
-
- White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. - PubMed
-
- Hale V, Keasling JD, Renninger N, Diagana TT. Microbially Derived Artemisinin: A Biotechnology Solution to the Global Problem of Access to Affordable Antimalarial Drugs. Am J Trop Med Hyg. 2007;77:198–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

