Casein kinase II contributes to the synergistic effects of BMP7 and BDNF on Smad 1/5/8 phosphorylation in septal neurons under hypoglycemic stress
- PMID: 19222702
- PMCID: PMC2720619
- DOI: 10.1111/j.1471-4159.2009.05990.x
Casein kinase II contributes to the synergistic effects of BMP7 and BDNF on Smad 1/5/8 phosphorylation in septal neurons under hypoglycemic stress
Abstract
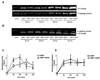
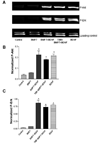
The combination of bone morphogenetic protein 7 (BMP7) and neurotrophins (e.g. brain-derived neurotrophic factor, BDNF) protects septal neurons during hypoglycemic stress. We investigated the signaling mechanisms underlying this synergistic protection. BMP7 (5 nM) increased phosphorylation and nuclear translocation of BMP-responsive Smads 1/5/8 within 30 min in cultures of rat embryonic septal neurons. BDNF (100 ng/mL) enhanced the BMP7-induced increase in phospho-Smad levels in both nucleus and cytoplasm; this effect was more pronounced after a hypoglycemic stress. BDNF increased both Akt and Erk phosphorylation, but pharmacological blockade of these kinase pathways (with wortmannin and U0126, respectively) did not reduce the Smad phosphorylation produced by the BMP7 + BDNF combination. Inhibitors of casein kinase II (CK2) activity reduced the (BMP7 + BDNF)-induced Smad phosphorylation, and this trophic factor combination increased CK2 activity in hypoglycemic cultures. These findings suggest that BDNF can increase BMP-dependent Smad phosphorylation via a mechanism requiring CK2.
Figures





References
-
- Althini S, Usoskin D, Kylberg A, Kaplan PL, Ebendal T. Blocked MAP kinase activity selectively enhances neurotrophic growth responses. Mol. Cell. Neurosci. 2004;25(2):345–354. - PubMed
-
- Arevalo MA, Rodriguez-Tebar A. Activation of casein kinase II and inhibition of phosphatase and tensin homologue deleted on chromosome 10 phosphatase by nerve growth factor/p75NTR inhibit glycogen synthase kinase-3beta and stimulate axonal growth. Mol. Biol. Cell. 2006;17(8):3369–3377. - PMC - PubMed
-
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27(2):265–277. - PubMed
-
- Bengtsson H, Soderstrom S, Kylberg A, Charette MF, Ebendal T. Potentiating interactions between morphogenetic protein and neurotrophic factors in developing neurons. J. Neurosci. Res. 1998;53(5):559–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

