In vitro glutaminase regulation and mechanisms of glutamate generation in HIV-1-infected macrophage
- PMID: 19222703
- PMCID: PMC2668921
- DOI: 10.1111/j.1471-4159.2009.05989.x
In vitro glutaminase regulation and mechanisms of glutamate generation in HIV-1-infected macrophage
Abstract
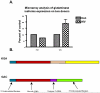
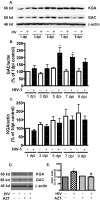
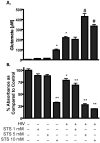
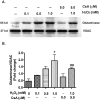
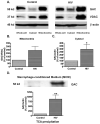
Mononuclear phagocyte (MP, macrophages and microglia) dysfunction plays a significant role in the pathogenesis of HIV-1-associated dementia (HAD) through the production and release of soluble neurotoxic factors including glutamate. Glutamate production is greatly increased following HIV-1 infection of cultured MP, a process dependent upon the glutamate-generating enzyme glutaminase. Glutaminase inhibition was previously found to significantly decrease macrophage-mediated neurotoxicity. Potential mechanisms of glutaminase-mediated excitotoxicity including enzyme up-regulation, increased enzyme activity and glutaminase localization were investigated in this report. RNA and protein analysis of HIV-infected human primary macrophage revealed up-regulation of the glutaminase isoform GAC, yet identified no changes in the kidney-type glutaminase isoform over the course of infection. Glutaminase is a mitochondrial protein, but was found to be released into the cytosol and extracellular space following infection. This released enzyme is capable of rapidly converting the abundant extracellular amino acid glutamine into excitotoxic levels of glutamate in an energetically favorable process. These findings support glutaminase as a potential component of the HAD pathogenic process and identify a possible therapeutic avenue for the treatment of neuroinflammatory states such as HAD.
Figures


References
-
- Belmadani A, Zou J, Schipma MJ, Neafsey EJ, Collins MA. Ethanol pre-exposure suppresses HIV-1 glycoprotein 120-induced neuronal degeneration by abrogating endogenous glutamate/Ca(2+)-mediated neurotoxicity. Neuroscience. 2001;104:769–781. - PubMed
-
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. - PubMed
-
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

