Transmembrane voltage regulates binding of annexin V and lactadherin to cells with exposed phosphatidylserine
- PMID: 19222854
- PMCID: PMC2653547
- DOI: 10.1186/1471-2091-10-5
Transmembrane voltage regulates binding of annexin V and lactadherin to cells with exposed phosphatidylserine
Abstract
Background: Cells expose phosphatidylserine during apoptosis. The voltage across the plasma membrane also decreases or disappears during apoptosis, but the physiological significance of this is unknown.
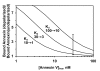
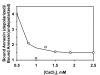
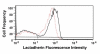
Results: Here we show that transmembrane potential regulates membrane binding of two unrelated proteins that recognize exposed phosphatidylserine on apoptotic cells. In Jurkat T leukemia cells and K562 promyelocytic leukemia cells undergoing apoptosis, extracellular binding of annexin V was increased by decreasing membrane potential in a dose-dependent manner. Studies with phospholipid vesicles showed that the effect was mediated via an increase in binding affinity. The effect was independent of the apoptotic stimulus. The same phenomenon occurred with lactadherin, a structurally unrelated protein that also binds to apoptotic cells via phosphatidylserine and is essential for in vivo clearance of dying cells.
Conclusion: Alterations in membrane potential regulate the binding of annexin V and lactadherin to cell membranes, and may also influence the membrane binding of other classes of phosphatidylserine-binding proteins.
Figures


Similar articles
-
Lactadherin binding and phosphatidylserine expression on cell surface-comparison with annexin A5.Transl Res. 2006 Jul;148(1):19-25. doi: 10.1016/j.lab.2006.03.006. Transl Res. 2006. PMID: 16887494
-
Measurement of phosphatidylserine exposure during storage of platelet concentrates using the novel probe lactadherin: a comparison study with annexin V.Transfusion. 2009 Jan;49(1):99-107. doi: 10.1111/j.1537-2995.2008.01933.x. Epub 2008 Oct 2. Transfusion. 2009. PMID: 18954406
-
Measurement of annexin V uptake and lactadherin labeling for the quantification of apoptosis in adherent Tca8113 and ACC-2 cells.Braz J Med Biol Res. 2008 Sep;41(9):750-7. doi: 10.1590/s0100-879x2008000900002. Braz J Med Biol Res. 2008. PMID: 18820763
-
Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization.Biochim Biophys Acta. 2008 Jun;1783(6):953-63. doi: 10.1016/j.bbamcr.2008.01.030. Epub 2008 Feb 20. Biochim Biophys Acta. 2008. PMID: 18334229 Review.
-
Lactadherin: An unappreciated haemostasis regulator and potential therapeutic agent.Vascul Pharmacol. 2018 Feb;101:21-28. doi: 10.1016/j.vph.2017.11.006. Epub 2017 Nov 21. Vascul Pharmacol. 2018. PMID: 29169950 Review.
Cited by
-
Pharmacokinetics of the phosphatidylserine tracers 99mTc-lactadherin and 99mTc-annexin V in pigs.EJNMMI Res. 2013 Mar 9;3(1):15. doi: 10.1186/2191-219X-3-15. EJNMMI Res. 2013. PMID: 23497537 Free PMC article.
-
Quantitative analysis of annexin V-membrane interaction by flow cytometry.Eur Biophys J. 2015 Jul;44(5):325-36. doi: 10.1007/s00249-015-1026-9. Epub 2015 Apr 29. Eur Biophys J. 2015. PMID: 25921613
-
Multifunctional Heterometallic IrIII -AuI Probes as Promising Anticancer and Antiangiogenic Agents.Chemistry. 2021 Jul 7;27(38):9885-9897. doi: 10.1002/chem.202100707. Epub 2021 May 29. Chemistry. 2021. PMID: 33860585 Free PMC article.
-
Functional Interactions between BKCaα-Subunit and Annexin A5: Implications in Apoptosis.Oxid Med Cell Longev. 2016;2016:1607092. doi: 10.1155/2016/1607092. Epub 2016 Sep 25. Oxid Med Cell Longev. 2016. PMID: 27738490 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

