Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain
- PMID: 19223010
- PMCID: PMC2677992
- DOI: 10.1016/j.neuroscience.2009.02.023
Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain
Abstract
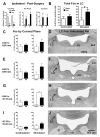
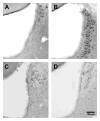
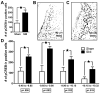
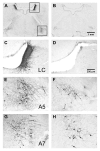
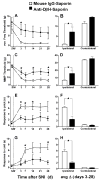
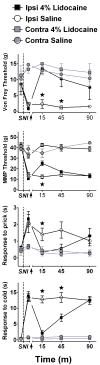
Current theories of neuropathic hypersensitivity include an imbalance of supraspinal inhibition and facilitation. Our overall hypothesis is that the locus coeruleus (LC), classically interpreted as a source of pain inhibition, may paradoxically result in facilitation after tibial and common peroneal nerve transection (spared sural nerve injury--SNI). We first tested the hypothesis that non-noxious tactile hind paw stimulation of the spared sural innervation territory increases neuronal activity in the LC in male rats. We observed a bilateral increase in the stimulus-evoked expression of transcription factors Fos and phosphorylated CREB (pCREB) in LC after SNI but not sham surgery; these markers of neuronal activity correlated with the intensity of tactile allodynia. We next tested the hypothesis that noradrenergic neurons contribute to the development of neuropathic pain. To selectively destroy these neurons, we delivered antidopamine-beta-hydroxylase saporin (anti-DbetaH-saporin) into the i.c.v. space 2 weeks before SNI. We found that anti-DbetaH-saporin, but not an IgG-saporin control, reduced behavioral signs of tactile allodynia, mechanical hyperalgesia, and cold allodynia from 3 to 28 days. after SNI. Our final experiment tested the hypothesis that the LC contributes to the maintenance of neuropathic pain. We performed SNI, waited 2 weeks for maximal allodynia and hyperalgesia to develop, and then administered the local anesthetic lidocaine (4%) directly into the LC parenchyma. Lidocaine reduced all behavioral signs of neuropathic pain in a reversible manner, suggesting that the LC contributes to pain facilitation. We conclude that, in addition to its well-known inhibition of acute and inflammatory pain, the LC facilitates the development and maintenance of neuropathic pain in the SNI model. Further studies are needed to determine the facilitatory pathways emanating from the LC.
Figures

Similar articles
-
GABA-A receptor activity in the noradrenergic locus coeruleus drives trigeminal neuropathic pain in the rat; contribution of NAα1 receptors in the medial prefrontal cortex.Neuroscience. 2016 Oct 15;334:148-159. doi: 10.1016/j.neuroscience.2016.08.005. Epub 2016 Aug 9. Neuroscience. 2016. PMID: 27520081 Free PMC article.
-
Histamine H4 receptor stimulation in the locus coeruleus attenuates neuropathic pain by promoting the coeruleospinal noradrenergic inhibitory pathway.Eur J Pharmacol. 2020 Feb 5;868:172859. doi: 10.1016/j.ejphar.2019.172859. Epub 2019 Dec 14. Eur J Pharmacol. 2020. PMID: 31843515
-
Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain.J Neurosci. 2005 Jul 13;25(28):6658-66. doi: 10.1523/JNEUROSCI.1490-05.2005. J Neurosci. 2005. PMID: 16014727 Free PMC article.
-
Exploration of supraspinal mechanisms in effects of spinal cord stimulation: role of the locus coeruleus.Neuroscience. 2013 Dec 3;253:426-34. doi: 10.1016/j.neuroscience.2013.09.006. Epub 2013 Sep 11. Neuroscience. 2013. PMID: 24036376
-
Activation of glutamate transporters in the locus coeruleus paradoxically activates descending inhibition in rats.Brain Res. 2010 Mar 4;1317:80-6. doi: 10.1016/j.brainres.2009.12.086. Epub 2010 Jan 6. Brain Res. 2010. PMID: 20059984 Free PMC article.
Cited by
-
The noradrenergic locus coeruleus as a chronic pain generator.J Neurosci Res. 2017 Jun;95(6):1336-1346. doi: 10.1002/jnr.23956. Epub 2016 Sep 29. J Neurosci Res. 2017. PMID: 27685982 Free PMC article. Review.
-
Alterations in the cholinergic system of brain stem neurons in a mouse model of Rett syndrome.Am J Physiol Cell Physiol. 2014 Sep 15;307(6):C508-20. doi: 10.1152/ajpcell.00035.2014. Epub 2014 Jul 9. Am J Physiol Cell Physiol. 2014. PMID: 25009110 Free PMC article.
-
Brainstem Pain-Control Circuitry Connectivity in Chronic Neuropathic Pain.J Neurosci. 2018 Jan 10;38(2):465-473. doi: 10.1523/JNEUROSCI.1647-17.2017. Epub 2017 Nov 24. J Neurosci. 2018. PMID: 29175957 Free PMC article.
-
Neuro-Nutritional Approach to Neuropathic Pain Management: A Critical Review.Nutrients. 2025 Apr 29;17(9):1502. doi: 10.3390/nu17091502. Nutrients. 2025. PMID: 40362812 Free PMC article. Review.
-
Spinal dopaminergic projections control the transition to pathological pain plasticity via a D1/D5-mediated mechanism.J Neurosci. 2015 Apr 22;35(16):6307-17. doi: 10.1523/JNEUROSCI.3481-14.2015. J Neurosci. 2015. PMID: 25904784 Free PMC article.
References
-
- Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the patter of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69:101–110. - PubMed
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42.
-
- Al-Adawi S, Dawe GS, Bonner A, Stephenson JD, Zarei M. Central noradrenergic blockade prevents autotomy in rat: implication for pharmacological prevention of postdenervation pain syndrome. Brain Res Bull. 2002;57:581–586. - PubMed
-
- Aloisi AM, Steenbergen HL, van de Poll NE, Farabollini F. Sex-dependent effects of restraint on nociception and pituitary-adrenal hormones in the rat. Physiology and Behavior. 1994;55:789–793. - PubMed
-
- Aston-Jones G. Locus Coeruleus, A5 and A7 Noradrenergic Cell Groups. In: Paxinos G, editor. The Rat Nervous System. China: Elsevier; 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

