Transforming growth factor beta depletion is the primary determinant of Smad signaling kinetics
- PMID: 19223462
- PMCID: PMC2668365
- DOI: 10.1128/MCB.01443-08
Transforming growth factor beta depletion is the primary determinant of Smad signaling kinetics
Erratum in
-
Correction for Clarke et al., "Transforming Growth Factor β Depletion Is the Primary Determinant of Smad Signaling Kinetics".Mol Cell Biol. 2021 Jul 23;41(8):e0025521. doi: 10.1128/MCB.00255-21. Epub 2021 Jul 23. Mol Cell Biol. 2021. PMID: 34296951 Free PMC article. No abstract available.
Abstract
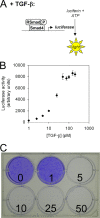
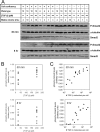
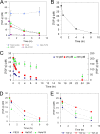
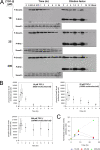
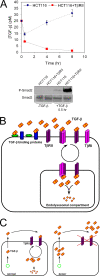
A cell's decision to growth arrest, apoptose, or differentiate in response to transforming growth factor beta (TGF-beta) superfamily ligands depends on the ligand concentration. How cells sense the concentration of extracellular bioavailable TGF-beta remains poorly understood. We therefore undertook a systematic quantitative analysis of how TGF-beta ligand concentration is transduced into downstream phospho-Smad2 kinetics, and we found that the rate of TGF-beta ligand depletion is the principal determinant of Smad signal duration. TGF-beta depletion is caused by two mechanisms: (i) cellular uptake of TGF-beta by a TGF-beta type II receptor-dependent mechanism and (ii) reversible binding of TGF-beta to the cell surface. Our results indicate that cells sense TGF-beta dose by depleting TGF-beta via constitutive TGF-beta type II receptor trafficking processes. Our results also have implications for the role of the TGF-beta type II receptor in disease, as tumor cells harboring TGF-beta type II receptor mutations exhibit impaired TGF-beta depletion, which may contribute to the overproduction of TGF-beta and a consequently poor prognosis in cancer.
Figures


References
-
- Abe, M., J. G. Harpel, C. N. Metz, I. Nunes, D. J. Loskutoff, and D. B. Rifkin. 1994. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216276-284. - PubMed
-
- Afrakhte, M., A. Moren, S. Jossan, S. Itoh, K. Sampath, B. Westermark, C. H. Heldin, N. E. Heldin, and P. ten Dijke. 1998. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem. Biophys. Res. Commun. 249505-511. - PubMed
-
- Ashe, H. L., and J. Briscoe. 2006. The interpretation of morphogen gradients. Development 133385-394. - PubMed
-
- Bangi, E., and K. Wharton. 2006. Dual function of the Drosophila Alk1/Alk2 ortholog Saxophone shapes the Bmp activity gradient in the wing imaginal disc. Development 1333295-3303. - PubMed
-
- Belenkaya, T. Y., C. Han, D. Yan, R. J. Opoka, M. Khodoun, H. Liu, and X. Lin. 2004. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell 119231-244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
