Small-molecule activation of p53 blocks hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in vivo and leads to tumor cell apoptosis in normoxia and hypoxia
- PMID: 19223463
- PMCID: PMC2663300
- DOI: 10.1128/MCB.00959-08
Small-molecule activation of p53 blocks hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in vivo and leads to tumor cell apoptosis in normoxia and hypoxia
Abstract
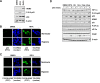
The p53 tumor suppressor protein negatively regulates hypoxia-inducible factor 1alpha (HIF-1alpha). Here, we show that induction of p53 by the small-molecule RITA (reactivation of p53 and induction of tumor cell apoptosis) [2,5-bis(5-hydroxymethyl-2-thienyl) furan] (NSC-652287) inhibits HIF-1alpha and vascular endothelial growth factor expression in vivo and induces significant tumor cell apoptosis in normoxia and hypoxia in p53-positive cells. RITA has been proposed to stabilize p53 by inhibiting the p53-HDM2 interaction. However, induction of p53 alone was insufficient to block HIF-1alpha induced in hypoxia and has previously been shown to require additional stimuli, such as DNA damage. Here, we identify a new mechanism of action for RITA: RITA activates a DNA damage response, resulting in phosphorylation of p53 and gammaH2AX in vivo. Unlike other DNA damage response-inducing agents, RITA treatment of cells induced a p53-dependent increase in phosphorylation of the alpha subunit of eukaryotic initiation factor 2, requiring PKR-like endoplasmic reticulum kinase activity, and led to the subsequent downregulation of HIF-1alpha and p53 target proteins, including HDM2 and p21. Through the identification of a new mechanism of action for RITA, our study uncovers a novel link between the DNA damage response-p53 pathway and the protein translational machinery.
Figures






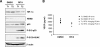
References
-
- Achison, M., and T. R. Hupp. 2003. Hypoxia attenuates the p53 response to cellular damage. Oncogene 223431-3440. - PubMed
-
- Beuvink, I., A. Boulay, S. Fumagalli, F. Zilbermann, S. Ruetz, T. O'Reilly, F. Natt, J. Hall, H. A. Lane, and G. Thomas. 2005. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 120747-759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
