Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat
- PMID: 19223593
- PMCID: PMC2651236
- DOI: 10.1073/pnas.0809620106
Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat
Abstract
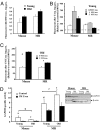
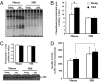
The widely accepted oxidative stress theory of aging postulates that aging results from accumulation of oxidative damage. Surprisingly, data from the longest-living rodent known, naked mole-rats [MRs; mass 35 g; maximum lifespan (MLSP) > 28.3 years], when compared with mice (MLSP 3.5 years) exhibit higher levels of lipid peroxidation, protein carbonylation, and DNA oxidative damage even at a young age. We hypothesize that age-related changes in protein structural stability, oxidation, and degradation are abrogated over the lifespan of the MR. We performed a comprehensive study of oxidation states of protein cysteines [both reversible (sulfenic, disulfide) and indirectly irreversible (sulfinic/sulfonic acids)] in liver from young and old C57BL/6 mice (6 and 28 months) and MRs (2 and >24 years). Furthermore, we compared interspecific differences in urea-induced protein unfolding and ubiquitination and proteasomal activity. Compared with data from young mice, young MRs have 1.6 times as much free protein thiol groups and similar amounts of reversible oxidative damage to cysteine. In addition, they show less urea-induced protein unfolding, less protein ubiquitination, and higher proteasome activity. Mice show a significant age-related increase in cysteine oxidation and higher levels of ubiquitination. In contrast, none of these parameters were significantly altered over 2 decades in MRs. Clearly MRs have markedly attenuated age-related accrual of oxidation damage to thiol groups and age-associated up-regulation of homeostatic proteolytic activity. These pivotal mechanistic interspecies differences may contribute to the divergent aging profiles and strongly implicate maintenance of protein stability and integrity in successful aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Buffenstein R, Jarvis J U. The naked mole rat: A new record for the oldest living rodent. Sci Aging Knowledge Environ. 2002;2002:pe7. - PubMed
-
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: Insights from a successfully aging species. J Comp Physiol B. 2008;178:439–445. - PubMed
-
- O'Connor TP, Lee A, Jarvis JU, Buffenstein R. Prolonged longevity in naked mole-rats: Age-related changes in metabolism, body composition, and gastrointestinal function. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:835–842. - PubMed
-
- Csiszar A, et al. Vascular aging in the longest-living rodent, the naked mole-rat. Am J Physiol. 2007;293:H919–H927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

