The role of calsenilin/DREAM/KChIP3 in contextual fear conditioning
- PMID: 19223600
- PMCID: PMC2661250
- DOI: 10.1101/lm.1261709
The role of calsenilin/DREAM/KChIP3 in contextual fear conditioning
Abstract
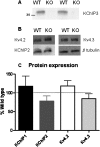
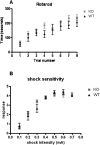
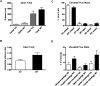
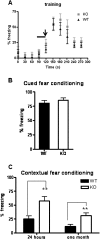
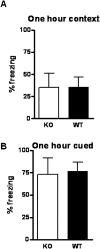
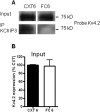
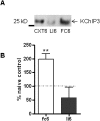
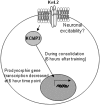
Potassium channel interacting proteins (KChIPs) are members of a family of calcium binding proteins that interact with Kv4 potassium (K(+)) channel primary subunits and also act as transcription factors. The Kv4 subunit is a primary K(+) channel pore-forming subunit, which contributes to the somatic and dendritic A-type currents throughout the nervous system. These A-type currents play a key role in the regulation of neuronal excitability and dendritic processing of incoming synaptic information. KChIP3 is also known as calsenilin and as the transcription factor, downstream regulatory element antagonist modulator (DREAM), which regulates a number of genes including prodynorphin. KChIP3 and Kv4 primary channel subunits are highly expressed in hippocampus, an area of the brain important for learning and memory. Through its various functions, KChIP3 may play a role in the regulation of synaptic plasticity and learning and memory. We evaluated the role of KChIP3 in a hippocampus-dependent memory task, contextual fear conditioning. Male KChIP3 knockout (KO) mice showed significantly enhanced memory 24 hours after training as measured by percent freezing. In addition, we found that membrane association and interaction with Kv4.2 of KChIP3 protein was significantly decreased and nuclear KChIP3 expression was increased six hours after the fear conditioning training paradigm with no significant change in KChIP3 mRNA. In addition, prodynorphin mRNA expression was significantly decreased six hours after fear conditioning training in wild-type (WT) but not in KO animals. These data suggest a role for regulation of gene expression by KChIP3/DREAM/calsenilin in consolidation of contextual fear conditioning memories.
Figures

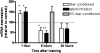
References
-
- An W.F., Bowlby M.R., Betty M., Cao J., Ling H.P., Mendoza G., Hinson J.W., Mattsson K.I., Strassle B.W., Trimmer J.S., et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
-
- Bahring R., Dannenberg J., Peters H.C., Leicher T., Pongs O., Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating. J. Biol. Chem. 2001;276:23888–23894. - PubMed
-
- Birnbaum S.G., Varga A.W., Yuan L.L., Anderson A.E., Sweatt J.D., Schrader L.A. Structure and function of Kv4-family transient potassium channels. Physiol. Rev. 2004;84:803–833. - PubMed
-
- Boland L.M., Jiang M., Lee S.Y., Fahrenkrug S.C., Harnett M.T., O'Grady S.M. Functional properties of a brain-specific NH2-terminally spliced modulator of Kv4 channels. Am. J. Physiol. Cell Physiol. 2003;285:C161–C170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
