ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification
- PMID: 19223621
- PMCID: PMC2681496
- DOI: 10.1128/AAC.01596-08
ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification
Abstract
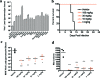
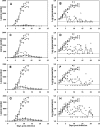
Therapeutics for the treatment of pathogenic orthopoxvirus infections are being sought. In the absence of patients with disease, animal models of orthopoxvirus disease are essential for evaluation of the efficacies of antiviral drugs and establishment of the appropriate dose and duration of human therapy. Infection of nonhuman primates (NHP) by the intravenous injection of monkeypox virus has been used to evaluate a promising therapeutic drug candidate, ST-246. ST-246 administered at 3 days postinfection (which corresponds to the secondary viremia stage of disease) at four different doses (from 100 mg/kg of body weight down to 3 mg/kg) once a day for 14 days was able to offer NHP 100% protection from a lethal infection with monkeypox virus and reduce the viral load and lesion formation. In NHP, the administration of ST-246 at a dose of 10 mg/kg/day for 14 days resulted in levels of blood exposure comparable to the levels attained in humans administered 400 mg in the fed state. These results suggest that administration of an oral dosage of 400 mg once daily for 14 days will be effective for the prevention or treatment of smallpox or monkeypox infections in humans.
Figures
References
-
- Breman, J. G., and D. A. Henderson. 2002. Diagnosis and management of smallpox. N. Engl. J. Med. 346:1300-1308. - PubMed
-
- Duraffour, S., R. Snoeck, R. de Vos, J. J. van den Oord, J. M. Crance, D. Garin, D. E. Hruby, R. Jordan, E. De Clercq, and G. Andrei. 2007. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antivir. Ther. 12:1205-1216. - PubMed
-
- Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, J. C. Whitbeck, G. H. Cohen, R. J. Eisenberg, C. J. Hartmann, D. L. Jackson, D. A. Kulesh, M. J. Martinez, D. M. Miller, E. M. Mucker, J. D. Shamblin, S. H. Zwiers, J. W. Huggins, P. B. Jahrling, and B. Moss. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182-185. - PubMed
-
- Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

