Model-based approach to characterize efavirenz autoinduction and concurrent enzyme induction with carbamazepine
- PMID: 19223624
- PMCID: PMC2687212
- DOI: 10.1128/AAC.01120-08
Model-based approach to characterize efavirenz autoinduction and concurrent enzyme induction with carbamazepine
Abstract
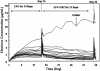
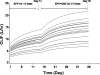
Characterization of the time course and magnitude of enzyme induction due to multiple inducers is important for interpretation of clinical data from drug-drug interaction studies. A population interaction model was developed to quantify efavirenz autoinduction and further induction with concurrent carbamazepine coadministration. Efavirenz concentration data in the absence and presence of carbamazepine following single- and multiple-dose oral administrations in healthy subjects were used for model development. The proposed model was able to describe the time-dependent efavirenz autoinduction and the further induction with carbamazepine when the agents were combined. The estimated population averages of efavirenz oral clearance were 5.5, 9.4, 14.4, and 16.7 liters/h on days 1, 14, and 35 and at steady state for the interaction, respectively, for efavirenz monotherapy for 2 weeks followed by the coadministration of carbamazepine for 3 weeks. The estimated times to 50% of the steady state for efavirenz autoinduction and for the induction resulting from the concurrent administration of efavirenz and carbamazepine were similar (around 10 to 12 days). With this model-based analysis, efavirenz exposures can be projected prior to and at the steady state of induction, allowing a better understanding of the time course and magnitude of enzyme induction.
Figures



References
-
- Adkins, J. C., and S. Noble. 1998. Efavirenz. Drugs 56:1055-1064. - PubMed
-
- Barrett, J. S., A. S. Joshi, T. M. Ludden, W. D. Fiske, and H. J. Pieniaszek, Jr. 2002. Population pharmacokinetic meta-analysis with efavirenz. Int. J. Clin. Pharmacol. Ther. 40:507-519. - PubMed
-
- Barrett, J. S., L. Labbe, and M. Pfister. 2005. Application and impact of population pharmacokinetics in the assessment of antiretroviral pharmacotherapy. Clin. Pharmacokinet. 44:591-625. - PubMed
-
- Beal, S. L., L. B. Sheiner, and A. J. Boeckmann (ed.). 1989-2006. NONMEM user's guides. Icon Development Solutions, Ellicott City, MD.
-
- Bernus, I., R. G. Dickinson, W. D. Hooper, and M. J. Eadie. 1996. Dose-dependent metabolism of carbamazepine in humans. Epilepsy Res. 24:163-172. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

