Role of adipocyte-derived apoE in modulating adipocyte size, lipid metabolism, and gene expression in vivo
- PMID: 19223650
- PMCID: PMC2763826
- DOI: 10.1152/ajpendo.90964.2008
Role of adipocyte-derived apoE in modulating adipocyte size, lipid metabolism, and gene expression in vivo
Abstract
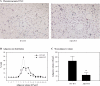
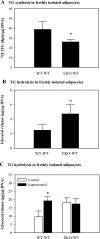
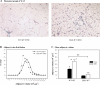
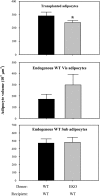
Adipocytes isolated from apolipoprotein E (apoE)-knockout (EKO) mice display alterations in triglyceride (TG) metabolism and gene expression. The present studies were undertaken to evaluate the impact of endogenously produced adipocyte apoE on these adipocyte parameters in vivo, independent of the profoundly disturbed metabolic milieu of EKO mice. Adipose tissue from wild-type (WT) or EKO mice was transplanted into WT recipients, which were then fed chow or high-fat diet for 8-10 wk. After a chow diet, freshly isolated transplanted EKO adipocytes were significantly (P < 0.05) smaller (70%) than transplanted WT adipocytes and displayed significantly lower rates of TG synthesis and higher rates of TG hydrolysis. Transplanted EKO adipocytes also had higher mRNA levels for adiponectin, perilipin, and genes coding for enzymes in the fatty acid oxidation pathway and lower levels of caveolin. After a high-fat diet and consequent increase in circulating lipid and apoE levels, transplanted WT adipocyte size increased by 106 x 10(3) microm(3), whereas EKO adipocyte size increased only by 19 x 10(3) microm(3). Endogenous host adipose tissue harvested from WT recipients of transplanted WT or EKO adipose tissue did not demonstrate any difference in adipocyte size. Consistent with the in vivo observations, EKO adipocytes synthesized less TG when incubated with apoE-containing TG-rich lipoproteins than WT adipocytes. Our results establish a novel in vivo role for endogenously produced apoE, distinct from circulating apoE, in modulation of adipocyte TG metabolism and gene expression. They support a model in which endogenously produced adipocyte apoE facilitates adipocyte lipid acquisition from circulating TG-rich lipoproteins.
Figures

Similar articles
-
Adipose tissue depot-specific differences in adipocyte apolipoprotein E expression.Metabolism. 2011 Dec;60(12):1692-701. doi: 10.1016/j.metabol.2011.04.012. Epub 2011 Jun 12. Metabolism. 2011. PMID: 21664633 Free PMC article.
-
Mechanism for endogenously expressed ApoE modulation of adipocyte very low density lipoprotein metabolism: role in endocytic and lipase-mediated metabolic pathways.J Biol Chem. 2009 Nov 13;284(46):31512-22. doi: 10.1074/jbc.M109.004754. Epub 2009 Sep 18. J Biol Chem. 2009. PMID: 19767394 Free PMC article.
-
Hyperglycemia and advanced glycosylation end products suppress adipocyte apoE expression: implications for adipocyte triglyceride metabolism.Am J Physiol Endocrinol Metab. 2010 Oct;299(4):E615-23. doi: 10.1152/ajpendo.00273.2010. Epub 2010 Jul 20. Am J Physiol Endocrinol Metab. 2010. PMID: 20647555 Free PMC article.
-
Mouse models of lipodystrophy and their significance in understanding fat regulation.Curr Top Dev Biol. 2014;109:53-96. doi: 10.1016/B978-0-12-397920-9.00005-6. Curr Top Dev Biol. 2014. PMID: 24947236 Review.
-
Insights into the roles of Apolipoprotein E in adipocyte biology and obesity.Int J Obes (Lond). 2024 Sep;48(9):1205-1215. doi: 10.1038/s41366-024-01549-9. Epub 2024 Jun 5. Int J Obes (Lond). 2024. PMID: 38839985 Review.
Cited by
-
Human APOE4 Protects High-Fat and High-Sucrose Diet Fed Targeted Replacement Mice against Fatty Liver Disease Compared to APOE3.Aging Dis. 2024 Feb 1;15(1):259-281. doi: 10.14336/AD.2023.0530. Aging Dis. 2024. PMID: 37450924 Free PMC article.
-
Very low density lipoprotein receptor (VLDLR) expression is a determinant factor in adipose tissue inflammation and adipocyte-macrophage interaction.J Biol Chem. 2014 Jan 17;289(3):1688-703. doi: 10.1074/jbc.M113.515320. Epub 2013 Nov 29. J Biol Chem. 2014. PMID: 24293365 Free PMC article.
-
Apolipoprotein E synthesized by adipocyte and apolipoprotein E carried on lipoproteins modulate adipocyte triglyceride content.Lipids Health Dis. 2014 Aug 23;13:136. doi: 10.1186/1476-511X-13-136. Lipids Health Dis. 2014. PMID: 25148848 Free PMC article. Review.
-
Defective triglyceride biosynthesis in CETP-deficient SW872 cells.J Lipid Res. 2015 Sep;56(9):1669-78. doi: 10.1194/jlr.M056481. Epub 2015 Jul 22. J Lipid Res. 2015. PMID: 26203075 Free PMC article.
-
Adipose tissue depot-specific differences in adipocyte apolipoprotein E expression.Metabolism. 2011 Dec;60(12):1692-701. doi: 10.1016/j.metabol.2011.04.012. Epub 2011 Jun 12. Metabolism. 2011. PMID: 21664633 Free PMC article.
References
-
- Abildayeva K, Jansen PJ, Hirsch-Reinshagen V, Bloks VW, Bakker AH, Ramaekers FC, de Vente J, Groen AK, Wellington CL, Kuipers F, Mulder M. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J Biol Chem 281: 12799–12808, 2006. - PubMed
-
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96: 939–949, 2005. - PubMed
-
- Carmel JF, Tarnus E, Cohn JS, Bourdon E, Davignon J, Bernier L. High expression of apolipoprotein E impairs lipid storage and promotes cell proliferation in human adipocytes. J Cell Biochem 106: 608–617, 2009. - PubMed
-
- Chiba T, Nakazawa T, Yui K, Kaneko E, Shimokado K. VLDL induces adipocyte differentiation in ApoE-dependent manner. Arterioscler Thromb Vasc Biol 23: 1423–1429, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

