Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine
- PMID: 19223664
- PMCID: PMC2672872
- DOI: 10.1124/jpet.108.150151
Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine
Abstract
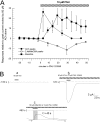
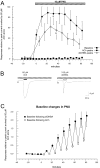
Nicotinic receptor activation is inextricably linked to desensitization. This duality affects our ability to develop useful therapeutics targeting nicotinic acetylcholine receptor (nAChR). Nicotine and some alpha7-selective experimental partial agonists produce a transient activation of alpha7 receptors followed by a period of prolonged residual inhibition or desensitization (RID). The object of the present study was to determine whether RID was primarily due to prolonged desensitization or due to channel block. To make this determination, we used agents that varied significantly in their production of RID and two alpha7-selective positive allosteric modulators (PAMs): 5-hydroxyindole (5HI), a type 1 PAM that does not prevent desensitization; and 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxanol-3-yl)-urea (PNU-120596), a type 2 PAM that reactivates desensitized receptors. The RID-producing compounds nicotine and 3-(2,4-dimethoxybenzylidene)anabaseine (diMeOBA) could obscure the potentiating effects of 5HI. However, through the use of nicotine, diMeOBA, and the RID-negative compound 3-(2,4-dihydroxybenzylidene)anabaseine (diOHBA) in combination with PNU-120596, we confirmed that diMeOBA produces short-lived channel block of alpha7 but that RID is because of the induction of a desensitized state that is stable in the absence of PNU-120596 and activated in the presence of PNU-120596. In contrast, diOHBA produced channel block but only readily reversible desensitization, whereas nicotine produced desensitization that could be converted into activation by PNU-120596 but no demonstrable channel block. Steady-state currents through receptors that would otherwise be desensitized could also be produced by the application of PNU-120596 in the presence of a physiologically relevant concentration of choline (60 microM), which may be significant for the therapeutic development of type 2 PAMs.
Figures









Similar articles
-
Point-to-point ligand-receptor interactions across the subunit interface modulate the induction and stabilization of conformational states of alpha7 nAChR by benzylidene anabaseines.Biochem Pharmacol. 2013 Mar 15;85(6):817-28. doi: 10.1016/j.bcp.2013.01.010. Epub 2013 Jan 23. Biochem Pharmacol. 2013. PMID: 23352650 Free PMC article.
-
Positive modulation of alpha7 nAChR responses in rat hippocampal interneurons to full agonists and the alpha7-selective partial agonists, 4OH-GTS-21 and S 24795.Neuropharmacology. 2009 Mar;56(4):821-30. doi: 10.1016/j.neuropharm.2009.01.011. Neuropharmacology. 2009. PMID: 19705574 Free PMC article.
-
Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes.Mol Pharmacol. 2007 Sep;72(3):715-24. doi: 10.1124/mol.107.035410. Epub 2007 Jun 12. Mol Pharmacol. 2007. PMID: 17565004
-
Silent agonists for α7 nicotinic acetylcholine receptors.Pharmacol Res. 2023 Apr;190:106736. doi: 10.1016/j.phrs.2023.106736. Epub 2023 Mar 20. Pharmacol Res. 2023. PMID: 36940890 Review.
-
Advances in the discovery of novel positive allosteric modulators of the alpha7 nicotinic acetylcholine receptor.Recent Pat CNS Drug Discov. 2007 Jun;2(2):99-106. doi: 10.2174/157488907780832751. Recent Pat CNS Drug Discov. 2007. PMID: 18221220 Review.
Cited by
-
Expeditious synthesis, enantiomeric resolution, and enantiomer functional characterization of (4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (4BP-TQS): an allosteric agonist-positive allosteric modulator of α7 nicotinic acetylcholine receptors.J Med Chem. 2013 Nov 14;56(21):8943-7. doi: 10.1021/jm401267t. Epub 2013 Oct 25. J Med Chem. 2013. PMID: 24090443 Free PMC article.
-
Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal alpha7 nicotinic acetylcholine receptor.EMBO J. 2009 Oct 7;28(19):3040-51. doi: 10.1038/emboj.2009.227. Epub 2009 Aug 20. EMBO J. 2009. PMID: 19696737 Free PMC article.
-
Single-channel and structural foundations of neuronal α7 acetylcholine receptor potentiation.J Neurosci. 2011 Sep 28;31(39):13870-9. doi: 10.1523/JNEUROSCI.2652-11.2011. J Neurosci. 2011. PMID: 21957249 Free PMC article.
-
An internally modulated, thermostable, pH-sensitive Cys loop receptor from the hydrothermal vent worm Alvinella pompejana.J Biol Chem. 2014 May 23;289(21):15130-40. doi: 10.1074/jbc.M113.525576. Epub 2014 Apr 9. J Biol Chem. 2014. PMID: 24719323 Free PMC article.
-
Differential immediate and sustained memory enhancing effects of alpha7 nicotinic receptor agonists and allosteric modulators in rats.PLoS One. 2011;6(11):e27014. doi: 10.1371/journal.pone.0027014. Epub 2011 Nov 9. PLoS One. 2011. PMID: 22096516 Free PMC article.
References
-
- Bertrand D, Bertrand S, Cassar S, Gubbins E, Li J, and Gopalakrishnan M (2008) Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2–M3 segment. Mol Pharmacol 74 1407-1416. - PubMed
-
- Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, Campbell JE, Decker MW, Donnelly-Roberts D, Elliott RL, Gopalakrishnan M, et al. (1997) Functional characterization of the novel nicotinic receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav 57 231-241. - PubMed
-
- de Fiebre CM, Meyer EM, Henry JC, Muraskin SI, Kem WR, and Papke RL (1995) Characterization of a family of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic α7/[125I]α-bungarotoxin receptor subtypes. Mol Pharmacol 47 164-171. - PubMed
-
- Farooqui AA and Horrocks LA (1994) Excitotoxicity and neurological disorders: involvement of membrane phospholipids. Int Rev Neurobiol 36 267-323. - PubMed
-
- Frazier CJ, Strowbridge BW, and Papke RL (2003) Nicotinic acetylcholine receptors on local circuit neurons in the dentate gyrus: a potential role in the regulation of granule cell excitability. J Neurophysiol 89 3018-3028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

