Copper(II) sulfamate: an efficient catalyst for the one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-ones and thiones
- PMID: 19223824
- PMCID: PMC6254017
- DOI: 10.3390/molecules14020763
Copper(II) sulfamate: an efficient catalyst for the one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-ones and thiones
Abstract
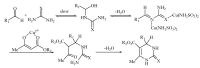
A simple, efficient procedure for the one-pot Biginelli condensation reaction of aldehydes, beta-ketoesters and urea or thiourea employing copper(II) sulfamate as a novel catalyst is described. Compared to the classical Biginelli reaction conditions, the present method has the advantages of good yields, short reaction times and experimental simplicity.
Figures
Similar articles
-
An efficient synthesis of 3,4-Dihydropyrimidin-2(1H)-ones and thiones catalyzed by a novel Brønsted acidic ionic liquid under solvent-free conditions.Molecules. 2015 Feb 26;20(3):3811-20. doi: 10.3390/molecules20033811. Molecules. 2015. PMID: 25730389 Free PMC article.
-
Copper nitrate catalyzed three-component one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones.Prep Biochem Biotechnol. 2009;39(4):372-9. doi: 10.1080/10826060903209596. Prep Biochem Biotechnol. 2009. PMID: 19739024
-
One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using CuBr2 as catalyst.Prep Biochem Biotechnol. 2006;36(4):375-81. doi: 10.1080/10826060600912682. Prep Biochem Biotechnol. 2006. PMID: 16971307
-
Recent developments in the synthesis and applications of dihydropyrimidin-2(1H)-ones and thiones.Mol Divers. 2018 May;22(2):405-446. doi: 10.1007/s11030-017-9806-z. Epub 2018 Jan 18. Mol Divers. 2018. PMID: 29349521 Review.
-
Recent progress in asymmetric Biginelli reaction.Mol Divers. 2013 May;17(2):389-407. doi: 10.1007/s11030-013-9439-9. Epub 2013 Apr 16. Mol Divers. 2013. PMID: 23588897 Review.
Cited by
-
Synthesis, spectral characterization and analgesic activity of 2-methylthio-1,4-dihydropyrimidines.Iran J Pharm Res. 2011 Fall;10(4):733-9. Iran J Pharm Res. 2011. PMID: 24250408 Free PMC article.
-
In Melting Points We Trust: A Review on the Misguiding Characterization of Multicomponent Reactions Adducts and Intermediates.Molecules. 2022 Nov 4;27(21):7552. doi: 10.3390/molecules27217552. Molecules. 2022. PMID: 36364380 Free PMC article. Review.
-
An efficient synthesis of 3,4-Dihydropyrimidin-2(1H)-ones and thiones catalyzed by a novel Brønsted acidic ionic liquid under solvent-free conditions.Molecules. 2015 Feb 26;20(3):3811-20. doi: 10.3390/molecules20033811. Molecules. 2015. PMID: 25730389 Free PMC article.
-
Magnetic core-shell Carrageenan moss/Fe3O4: a polysaccharide-based metallic nanoparticles for synthesis of pyrimidinone derivatives via Biginelli reaction.Chem Cent J. 2018 Oct 27;12(1):108. doi: 10.1186/s13065-018-0477-3. Chem Cent J. 2018. PMID: 30368625 Free PMC article.
-
New Efficient Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones Catalyzed by Benzotriazolium-Based Ionic Liquids under Solvent-Free Conditions.Molecules. 2016 Apr 7;21(4):462. doi: 10.3390/molecules21040462. Molecules. 2016. PMID: 27070558 Free PMC article.
References
-
- Kappe C.O. 100 Years of the Biginelli dihydropyridine synthesis. Tetrahedron. 1993;49:6937–6963. doi: 10.1016/S0040-4020(01)87971-0. - DOI
-
- Kappe C.O., Fabian W.M.F., Semones M.A. Conformational Analysis of 4-Aryl-Dihydropyrimidine Calcium Channel Modulators. A Comparison of Ab Initio, Semiempirical and X-Ray Crystallographic Studies. Tetrahedron. 1997;53:2803–2816. doi: 10.1016/S0040-4020(97)00022-7. - DOI
-
- Atwal K.S., Rovnyak G.C., Kimball S.D., Floyd D.M., Moreland S., Swanson B.N., Gougoutas D.Z., Schewartz J., Smillie K.M., Malley M.F. Dihydropyrimidine Calcium Channel Blockers. II. 3-Substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic Acid Esters as Potent Mimics of Dihydropyridines. J. Med. Chem. 1990;33:2629–2635. doi: 10.1021/jm00171a044. - DOI - PubMed
-
- Nagarathnam D., Miao S.W., Lagu B., Chiu G., Fang J., Murali Dhar T.G., Zhang J., Tyagarajan S., Marzabadi M.R., Zhang F., Wong W.C., Sun W., Tian D., Zhang J., Wetzel J.M., Forray C., Chang R.S.L., Broten T.P., Schorn T.W., Chen T.B., O'Malley S., Ransom R.W., Schneck K., Bendesky R., Harrell C.M., Gluchowski C. Design and Synthesis of Novel α1a Adrenoceptor-Selective Antagonists. 1. Structure-Activity Relationship in Dihydropyrimidinones. J. Med. Chem. 1999;42:4764–4777. doi: 10.1021/jm990200p. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources