Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver
- PMID: 19223863
- PMCID: PMC2835106
- DOI: 10.1038/mt.2008.307
Redundant and synergistic mechanisms control the sequestration of blood-born adenovirus in the liver
Abstract
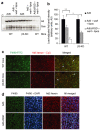
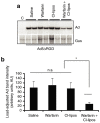
Human adenovirus (Ad) is a ubiquitous pathogen causing a wide range of diseases. Although the interactions of human Ad serotype 5 (Ad5) with susceptible cells in vitro are known in great detail, host factors controlling the tissue specificity of Ad5 infection in vivo remain poorly understood. Here, we analyzed the mechanisms of sequestration by the liver for blood-born human Ads and Ad5-based vectors. Our data suggest that several known mechanisms that lead to Ad5 sequestration by the liver become engaged in a redundant, sequential, and synergistic manner to ensure the rapid clearance of circulating virus particles from the blood. These mechanisms include (i) trapping of the virus by liver residential macrophages, Kupffer cells; (ii) Ad5 hepatocyte infection via blood factor-hexon interactions; and (iii) Ad5 penton RGD motif-mediated interactions with liver endothelial cells and hepatocytes, mediating virus retention in the space of Disse. More important, we show that when all of these mechanisms are simultaneously inactivated via mutations of Ad5 capsid proteins and pharmacological interventions, virus sequestration by the liver is markedly reduced. Therefore, our study is the first demonstration of the principal possibility of ablating the sequestration of blood-born Ad in the liver via specific inactivation of a defined set of mechanisms that control this process.
Figures
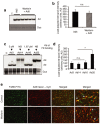
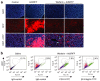
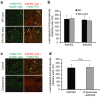
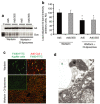

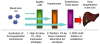
References
-
- Thomas CE, Ehrhardt A., and , Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Gen. 2003;4:346–358. - PubMed
-
- Alemany R, Suzuki K., and , Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81:2605–2609. - PubMed
-
- Worgall S, Wolff G, Falck-Pedersen E., and , Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. - PubMed
-
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Gaggar A, Shayakhmetov D., and , Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

