ICP0 inhibits the decrease of HSV amplicon-mediated transgene expression
- PMID: 19223864
- PMCID: PMC2835108
- DOI: 10.1038/mt.2008.306
ICP0 inhibits the decrease of HSV amplicon-mediated transgene expression
Abstract
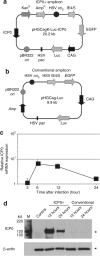
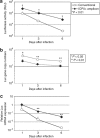
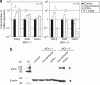
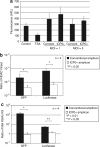
The herpes simplex virus (HSV) amplicon vector produces an initial host response that limits transgene expression. In this study, we hypothesized that restoration of the HSV gene infected cell protein (ICP0) into the amplicon could circumvent this host response and thus overcome silencing of encoded transgenes. To test this, we constructed an amplicon vector that encodes the ICP0 under control of its native promoter (ICP0+ amplicon). Expression of ICP0 was transient and, at a multiplicity of infection (MOI) of 1, did not significantly alter interferon (IFN)-based responses against the vector or cell kinetics/apoptosis of infected cells. Chromatin immunoprecipitation (ChIP) PCR analysis revealed that conventional amplicon DNA became associated with histone deacetylase 1 (HDAC1) immediately after infection, whereas ICP0+ amplicon DNA remained relatively unbound by HDAC1 for at least 72 hours after infection. Mice administered systemic ICP0+ amplicon exhibited significantly greater and more sustained transgene expression in their livers than did those receiving conventional amplicon, likely due to increased transcriptional or post-transcriptional activity rather than increased copy numbers of vector DNA. These findings indicate that restoration of ICP0 expression may be employed within HSV amplicon constructs to decrease transgene silencing in vitro and in vivo.
Figures

Similar articles
-
Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex.Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17134-9. doi: 10.1073/pnas.0707266104. Epub 2007 Oct 15. Proc Natl Acad Sci U S A. 2007. PMID: 17939992 Free PMC article.
-
Early STAT1 activation after systemic delivery of HSV amplicon vectors suppresses transcription of the vector-encoded transgene.Mol Ther. 2007 Nov;15(11):2017-26. doi: 10.1038/sj.mt.6300273. Epub 2007 Jul 24. Mol Ther. 2007. PMID: 17653098
-
The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27.J Virol. 1997 Jun;71(6):4614-25. doi: 10.1128/JVI.71.6.4614-4625.1997. J Virol. 1997. PMID: 9151855 Free PMC article.
-
Plasmid DNA sequences present in conventional herpes simplex virus amplicon vectors cause rapid transgene silencing by forming inactive chromatin.J Virol. 2006 Apr;80(7):3293-300. doi: 10.1128/JVI.80.7.3293-3300.2006. J Virol. 2006. PMID: 16537596 Free PMC article.
-
Role of herpes simplex virus ICP0 in the transactivation of genes introduced by infection or transfection: a reappraisal.J Virol. 2010 May;84(9):4222-8. doi: 10.1128/JVI.02585-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164233 Free PMC article.
Cited by
-
Non-replicative herpes simplex virus genomic and amplicon vectors for gene therapy - an update.Gene Ther. 2025 May;32(3):173-183. doi: 10.1038/s41434-024-00500-x. Epub 2024 Nov 12. Gene Ther. 2025. PMID: 39533042 Free PMC article. Review.
-
MyD88-dependent silencing of transgene expression during the innate and adaptive immune response to helper-dependent adenovirus.Hum Gene Ther. 2010 Mar;21(3):325-36. doi: 10.1089/hum.2009.155. Hum Gene Ther. 2010. PMID: 19824822 Free PMC article.
-
Constitutive and Inducible Innate Responses in Cells Infected by HSV-1-Derived Amplicon Vectors.Open Virol J. 2010 Jun 18;4:96-102. doi: 10.2174/1874357901004030096. Open Virol J. 2010. PMID: 20811588 Free PMC article.
-
Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs. adeno-associated viral vectors in vivo.Mol Ther. 2013 Apr;21(4):796-805. doi: 10.1038/mt.2012.277. Epub 2013 Jan 15. Mol Ther. 2013. PMID: 23319058 Free PMC article.
-
An HSV-1-H129 amplicon tracer system for rapid and efficient monosynaptic anterograde neural circuit tracing.Nat Commun. 2022 Dec 10;13(1):7645. doi: 10.1038/s41467-022-35355-6. Nat Commun. 2022. PMID: 36496505 Free PMC article.
References
-
- Wade-Martins R, Smith ER, Tyminski E, Chiocca EA., and , Saeki Y. An infectious transfer and expression system for genomic DNA loci in human and mouse cells. Nat Biotechnol. 2001;19:1067–1070. - PubMed
-
- Wade-Martins R, Saeki Y., and , Chiocca EA.Infectious delivery of a 135-kb LDLR genomic locus leads to regulated complementation of low-density lipoprotein receptor deficiency in human cells Mol Ther 20037604–612.5 Pt 1 - PubMed
-
- Inoue R, Moghaddam KA, Ranasinghe M, Saeki Y, Chiocca EA., and , Wade-Martins R. Infectious delivery of the 132 kb CDKN2A/CDKN2B genomic DNA region results in correctly spliced gene expression and growth suppression in glioma cells. Gene Ther. 2004;11:1195–1204. - PubMed
-
- Kasai K., and , Saeki Y. DNA-based methods to prepare helper virus-free herpes amplicon vectors and versatile design of amplicon vector plasmids. Curr Gene Ther. 2006;6:303–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

