Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element
- PMID: 19223867
- PMCID: PMC2835111
- DOI: 10.1038/mt.2009.1
Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element
Abstract
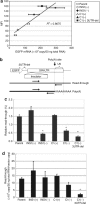
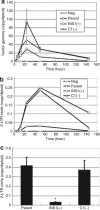
Hematopoietic cell gene therapy using retroviral vectors has achieved success in clinical trials. However, safety issues regarding vector insertional mutagenesis have emerged. In two different trials, vector insertion resulted in the transcriptional activation of proto-oncogenes. One strategy for potentially diminishing vector insertional mutagenesis is through the use of self-inactivating lentiviral vectors containing the 1.2-kb insulator element derived from the chicken beta-globin locus. However, use of this element can dramatically decrease both vector titer and transgene expression, thereby compromising its practical use. Here, we studied lentiviral vectors containing either the full-length 1.2-kb insulator or the smaller 0.25-kb core element in both orientations in the partially deleted long-terminal repeat. We show that use of the 0.25-kb core insulator rescued vector titer by alleviating a postentry block to reverse transcription associated with the 1.2-kb element. In addition, in an orientation-dependent manner, the 0.25-kb core element significantly increased transgene expression from an internal promoter due to improved transcriptional termination. This element also demonstrated barrier activity, reducing variability of expression due to position effects. As it is known that the 0.25-kb core insulator has enhancer-blocking activity, this particular insulated lentiviral vector design may be useful for clinical application.
Figures

Similar articles
-
Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector.Mol Ther. 2011 Jan;19(1):133-9. doi: 10.1038/mt.2010.218. Epub 2010 Oct 12. Mol Ther. 2011. PMID: 20940706 Free PMC article.
-
A chimeric HS4-SAR insulator (IS2) that prevents silencing and enhances expression of lentiviral vectors in pluripotent stem cells.PLoS One. 2014 Jan 6;9(1):e84268. doi: 10.1371/journal.pone.0084268. eCollection 2014. PLoS One. 2014. PMID: 24400083 Free PMC article.
-
Lentiviral Vectors Mediate Long-Term and High Efficiency Transgene Expression in HEK 293T cells.Int J Med Sci. 2015 May 15;12(5):407-15. doi: 10.7150/ijms.11270. eCollection 2015. Int J Med Sci. 2015. PMID: 26005375 Free PMC article.
-
Successful correction of the human Cooley's anemia beta-thalassemia major phenotype using a lentiviral vector flanked by the chicken hypersensitive site 4 chromatin insulator.Ann N Y Acad Sci. 2005;1054:238-49. doi: 10.1196/annals.1345.030. Ann N Y Acad Sci. 2005. PMID: 16339671 Review.
-
Progress toward the genetic treatment of the beta-thalassemias.Ann N Y Acad Sci. 2005;1054:78-91. doi: 10.1196/annals.1345.010. Ann N Y Acad Sci. 2005. PMID: 16339654 Review.
Cited by
-
Recent advances in gene therapy for thalassemia.J Pharm Bioallied Sci. 2012 Jul;4(3):194-201. doi: 10.4103/0975-7406.99020. J Pharm Bioallied Sci. 2012. PMID: 22923960 Free PMC article.
-
Hematopoietic stem cell engineering at a crossroads.Blood. 2012 Feb 2;119(5):1107-16. doi: 10.1182/blood-2011-09-349993. Epub 2011 Nov 17. Blood. 2012. PMID: 22096239 Free PMC article. Review.
-
RP58 controls neuron and astrocyte differentiation by downregulating the expression of Id1-4 genes in the developing cortex.EMBO J. 2012 Mar 7;31(5):1190-202. doi: 10.1038/emboj.2011.486. Epub 2012 Jan 10. EMBO J. 2012. PMID: 22234186 Free PMC article.
-
The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors.Hum Gene Ther. 2011 Jun;22(6):761-74. doi: 10.1089/hum.2010.233. Epub 2011 Mar 25. Hum Gene Ther. 2011. PMID: 21247248 Free PMC article. Review.
-
Assembly PCR synthesis of optimally designed, compact, multi-responsive promoters suited to gene therapy application.Sci Rep. 2016 Jul 8;6:29388. doi: 10.1038/srep29388. Sci Rep. 2016. PMID: 27387837 Free PMC article.
References
-
- Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. - PubMed
-
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. - PubMed
-
- Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. - PubMed
-
- Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. - PubMed
-
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

