Extended weekly dose-dense paclitaxel/carboplatin is feasible and active in heavily pre-treated platinum-resistant recurrent ovarian cancer
- PMID: 19223898
- PMCID: PMC2653750
- DOI: 10.1038/sj.bjc.6604914
Extended weekly dose-dense paclitaxel/carboplatin is feasible and active in heavily pre-treated platinum-resistant recurrent ovarian cancer
Abstract
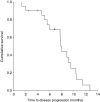
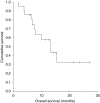
There is increasing evidence of the efficacy of dose-dense therapy in the management of platinum-resistant/refractory ovarian cancer. We report our experience of extended weekly carboplatin and paclitaxel in this population group. Twenty patients with platinum-resistant/refractory ovarian cancer received carboplatin AUC 3 and paclitaxel 70 mg m(-2) on day 1, 8, 15 q 4 weekly for six planned cycles. Toxicity was assessed using Common Toxicity Criteria. Response was evaluated using radiological and CA125 criteria. Median age was 61 years (range 40-74 years). Median number of prior therapies is three (range 1-8). Response rate was 60% by radiological criteria (RECIST) and 76% by CA125 assessment. Grade 3 toxicities consisted of neutropenia (29% of patients) and anaemia (5%). One patient experienced grade 4 neutropenia. No grade 3/4 thombocytopaenia was reported. Fatigue, nausea and peripheral neuropathy were the most frequent non-hematological side effects. Median progression-free survival was 7.9 months and overall survival was 13.3 months. The dynamics of response to dose-dense therapy were as rapid as with front-line therapy within the same patient. This dose-dense regimen can be extended to at least 18 weekly cycles over 6 months and is well tolerated with high response rates in heavily pre-treated, platinum-resistant ovarian cancer. It forms a highly active and tolerable cytotoxic scaffold to which molecular-targeted therapies can be added in platinum-resistant ovarian cancer.
Figures
Similar articles
-
Weekly dose-dense paclitaxel and carboplatin in recurrent ovarian carcinoma: a phase II trial.J Egypt Natl Canc Inst. 2014 Sep;26(3):139-45. doi: 10.1016/j.jnci.2014.05.001. Epub 2014 Jun 2. J Egypt Natl Canc Inst. 2014. PMID: 25150129 Clinical Trial.
-
Long-term results of weekly paclitaxel carboplatin induction therapy: an effective and well-tolerated treatment in patients with platinum-resistant ovarian cancer.Eur J Cancer. 2013 Apr;49(6):1254-63. doi: 10.1016/j.ejca.2012.11.027. Epub 2012 Dec 29. Eur J Cancer. 2013. PMID: 23276720 Clinical Trial.
-
Sustained platelet-sparing effect of weekly low dose paclitaxel allows effective, tolerable delivery of extended dose dense weekly carboplatin in platinum resistant/refractory epithelial ovarian cancer.BMC Cancer. 2011 Jul 11;11:289. doi: 10.1186/1471-2407-11-289. BMC Cancer. 2011. PMID: 21745358 Free PMC article. Clinical Trial.
-
Dose-dense chemotherapy with weekly paclitaxel and 3-weekly carboplatin for recurrent ovarian cancer.Taiwan J Obstet Gynecol. 2020 Jan;59(1):21-27. doi: 10.1016/j.tjog.2019.10.003. Taiwan J Obstet Gynecol. 2020. PMID: 32039795 Review.
-
What is the role of dose-dense therapy?Int J Gynecol Cancer. 2005 Nov-Dec;15 Suppl 3:233-40. doi: 10.1111/j.1525-1438.2005.00432.x. Int J Gynecol Cancer. 2005. PMID: 16343238 Review.
Cited by
-
Influence of CYP3A4 genotypes in the outcome of serous ovarian cancer patients treated with first-line chemotherapy: implication of a CYP3A4 activity profile.Int J Clin Exp Med. 2013 Aug 1;6(7):552-61. Print 2013. Int J Clin Exp Med. 2013. PMID: 23936594 Free PMC article.
-
Dose-dense chemotherapy improves mechanisms of antitumor immune response.Cancer Res. 2013 Jan 1;73(1):119-27. doi: 10.1158/0008-5472.CAN-12-2225. Epub 2012 Oct 29. Cancer Res. 2013. PMID: 23108141 Free PMC article. Clinical Trial.
-
Advances in tumor screening, imaging, and avatar technologies for high-grade serous ovarian cancer.Front Oncol. 2014 Nov 18;4:322. doi: 10.3389/fonc.2014.00322. eCollection 2014. Front Oncol. 2014. PMID: 25478323 Free PMC article. Review.
-
Combination of irinotecan and platinum for platinum-resistant or refractory recurrent ovarian cancers: A preliminary case series.Mol Clin Oncol. 2017 Jul;7(1):51-55. doi: 10.3892/mco.2017.1258. Epub 2017 May 12. Mol Clin Oncol. 2017. PMID: 28685075 Free PMC article.
-
Weekly paclitaxel in the treatment of recurrent ovarian cancer.Nat Rev Clin Oncol. 2010 Oct;7(10):575-82. doi: 10.1038/nrclinonc.2010.120. Epub 2010 Aug 3. Nat Rev Clin Oncol. 2010. PMID: 20683437 Review.
References
-
- Alvero AB, O’Malley D, Brown D, Kelly G, Garg M, Chen W, Rutherford T, Mor G (2006) Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer 106: 599–608 - PubMed
-
- Appleton K, Mackay HJ, Judson I, Plumb JA, McCormick C, Strathdee G, Lee C, Barrett S, Reade S, Jadayel D, Tang A, Bellenger K, Mackay L, Setanoians A, Schatzlein A, Twelves C, Kaye SB, Brown R (2007) Phase I and pharmacodynamic trial of the DNA methyltransferase inhibitor decitabine and carboplatin in solid tumors. J Clin Oncol 25: 4603–4609 - PubMed
-
- Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R, Taraboletti G (1996) The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res 2: 1843–1849 - PubMed
-
- Bolanos M, Borrega P, Gonzalez-Beca R, Arranz JA, Velasco A, Perez MM, Gomez-Bernal A, Martinez-Prado P, Pedro de Alcantara HS (2001) Weekly paclitaxel/carboplatinum as first-line chemotherapy in late relapses of epithelial ovarian cancer. A preliminary report on side effects. Proc Am Soc Clin Oncol 20, (abstr 870)
-
- Cadron I, Leunen K, Amant F, Van Gorp T, Neven P, Vergote I (2007) The ‘Leuven’ dose-dense paclitaxel/carboplatin regimen in patients with recurrent ovarian cancer. Gynecol Oncol 106: 354–361 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

