The role of slow potassium current in nerve conduction block induced by high-frequency biphasic electrical current
- PMID: 19224727
- PMCID: PMC2822242
- DOI: 10.1109/TBME.2008.2006013
The role of slow potassium current in nerve conduction block induced by high-frequency biphasic electrical current
Abstract
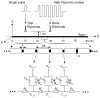
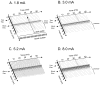
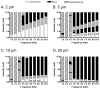
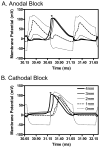
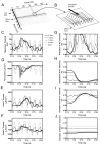
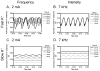
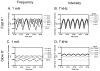
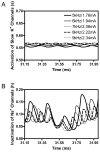
The role of slow potassium current in nerve conduction block induced by high-frequency biphasic electrical current was analyzed using a lumped circuit model of a myelinated axon based on the Schwarz-Reid-Bostock model. The results indicate that nerve conduction block at stimulation frequencies above 4 kHz is due to constant activation of both fast and slow potassium channels, but the block at stimulation frequencies below 4 kHz could be due to either anodal or cathodal dc block depending on the time of the action potential arriving at the block electrode. When stimulation frequency was above 4 kHz, the slow potassium current was about 3.5 to 6.5 times greater than the fast potassium current at blocking threshold, indicating that the slow potassium current played a more dominant role than the fast potassium current. The blocking location moved from the node under the blocking electrode to a nearby node as the stimulation intensity increased. This simulation study reveals that in mammalian myelinated axons, the slow potassium current probably plays a critical role in the nerve conduction block induced by high-frequency biphasic electrical current.
Figures

References
-
- Nashold B, Goldner J, Mullen J, Bright D. Long-term pain control by direct peripheral-nerve stimulation. J Bone Joint Surg. 1982;64(1):1–10. - PubMed
-
- Kilgore K, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput. 2004 May;42(3):394–406. - PubMed
-
- Tai C, Roppolo J, de Groat W. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J Urol. 2004;172(5):2069–2072. - PubMed
-
- Agnew W, McCreery D, editors. Prentice Hall Advanced Reference Series. Prentice Hall Biophysics and Bioengineering Series; Englewood Cliffs (New Jersey): 1990. Neural prostheses: Fundamental studies, ser.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

