Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup
- PMID: 19224846
- PMCID: PMC2668552
- DOI: 10.1200/JCO.2008.19.1684
Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup
Erratum in
- J Clin Oncol. 2009 May 1;27(13):2305
Abstract
Purpose: To determine if incorporation of an additional cytotoxic agent improves overall survival (OS) and progression-free survival (PFS) for women with advanced-stage epithelial ovarian carcinoma (EOC) and primary peritoneal carcinoma who receive carboplatin and paclitaxel.
Patients and methods: Women with stages III to IV disease were stratified by coordinating center, maximal diameter of residual tumor, and intent for interval cytoreduction and were then randomly assigned among five arms that incorporated gemcitabine, methoxypolyethylene glycosylated liposomal doxorubicin, or topotecan compared with carboplatin and paclitaxel. The primary end point was OS and was determined by pairwise comparison to the reference arm, with a 90% chance of detecting a true hazard ratio of 1.33 that limited type I error to 5% (two-tail) for the four comparisons.
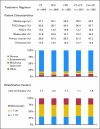
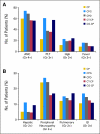
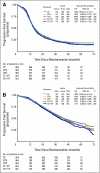
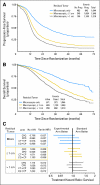
Results: Accrual exceeded 1,200 patients per year. An event-triggered interim analysis occurred after 272 events on the reference arm, and the study closed with 4,312 women enrolled. Arms were well balanced for demographic and prognostic factors, and 79% of patients completed eight cycles of therapy. There were no improvements in either PFS or OS associated with any experimental regimen. Survival analyses of groups defined by size of residual disease also failed to show experimental benefit in any subgroup.
Conclusion: Compared with standard paclitaxel and carboplatin, addition of a third cytotoxic agent provided no benefit in PFS or OS after optimal or suboptimal cytoreduction. Dual-stage, multiarm, phase III trials can efficiently evaluate multiple experimental regimens against a single reference arm. The development of new interventions beyond surgery and conventional platinum-based chemotherapy is required to additionally improve outcomes for women with advanced EOC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Triple cytotoxic therapy for advanced ovarian cancer: a failed application, not a failed strategy.J Clin Oncol. 2009 Mar 20;27(9):1355-8. doi: 10.1200/JCO.2008.20.8223. Epub 2009 Feb 17. J Clin Oncol. 2009. PMID: 19224837 No abstract available.
References
-
- ten Bokkel Huinink W, Lane SR, Ross GA, et al. Long-term survival in a phase III, randomised study of topotecan versus paclitaxel in advanced epithelial ovarian carcinoma. Ann Oncol. 2004;15:100–103. - PubMed
-
- Gordon AN, Tonda M, Sun S, et al. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1–8. - PubMed
-
- Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. - PubMed
-
- Greer BE, Bundy BN, Ozols RF, et al. Implications of second-look laparotomy in the context of optimally resected stage III ovarian cancer: A non-randomized comparison using an explanatory analysis: A Gynecologic Oncology Group study. Gynecol Oncol. 2005;99:71–79. - PubMed
-
- Rustin GJ, Timmers P, Nelstrop A, et al. Comparison of CA-125 and standard definitions of progression of ovarian cancer in the intergroup trial of cisplatin and paclitaxel versus cisplatin and cyclophosphamide. J Clin Oncol. 2006;24:45–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

