Synphilin-1A inhibits seven in absentia homolog (SIAH) and modulates alpha-synuclein monoubiquitylation and inclusion formation
- PMID: 19224863
- PMCID: PMC2670174
- DOI: 10.1074/jbc.M805990200
Synphilin-1A inhibits seven in absentia homolog (SIAH) and modulates alpha-synuclein monoubiquitylation and inclusion formation
Abstract
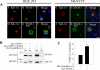
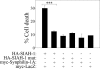
Parkinson disease (PD) is characterized by the presence of ubiquitylated inclusions and the death of dopaminergic neurons. Seven in absentia homolog (SIAH) is a ubiquitin-ligase that ubiquitylates alpha-synuclein and synphilin-1 and is present in Lewy bodies of PD patients. Understanding the mechanisms that regulate the ubiquitylation of PD-related proteins might shed light on the events involved in the formation of Lewy bodies and death of neurons. We show in this study that the recently described synphilin-1 isoform, synphilin-1A, interacts in vitro and in vivo with the ubiquitin-protein isopeptide ligase SIAH and regulates its activity toward alpha-synuclein and synphilin-1. SIAH promotes limited ubiquitylation of synphilin-1A that does not lead to its degradation by the proteasome. SIAH also increases the formation of synphilin-1A inclusions in the presence of proteasome inhibitors, supporting the participation of ubiquitylated synphilin-1A in the formation of Lewy body-like inclusions. Synphilin-1A/SIAH inclusions recruit PD-related proteins, such as alpha-synuclein, synphilin-1, Parkin, PINK1, and UCH-L1. We found that synphilin-1A robustly increases the steady-state levels of SIAH by decreasing its auto-ubiquitylation and degradation. In addition, synphilin-1A blocks the ubiquitylation and degradation of the SIAH substrates synphilin-1 and deleted in colon cancer protein. Furthermore, synphilin-1A strongly decreases the monoubiquitylation of alpha-synuclein by SIAH and the formation of alpha-synuclein inclusions, supporting a role for monoubiquitylation in alpha-synuclein inclusion formation. Our results suggest a novel function for synphilin-1A as a regulator of SIAH activity and formation of Lewy body-like inclusions.
Figures




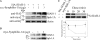



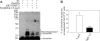
References
-
- Moore, D. J., West, A. B., Dawson, V. L., and Dawson, T. M. (2005) Annu. Rev. Neurosci. 28, 57-87 - PubMed
-
- Hardy, J., Cai, H., Cookson, M. R., Gwinn-Hardy, K., and Singleton, A. (2006) Ann. Neurol. 60, 389-398 - PubMed
-
- Lee, V. M., and Trojanowski, J. Q. (2006) Neuron 52, 33-38 - PubMed
-
- McNaught, K. S., and Jenner, P. (2001) Neurosci. Lett. 297, 191-194 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

