Atherosclerosis and matrix metalloproteinases: experimental molecular MR imaging in vivo
- PMID: 19224894
- PMCID: PMC2674553
- DOI: 10.1148/radiol.2511080539
Atherosclerosis and matrix metalloproteinases: experimental molecular MR imaging in vivo
Abstract
Purpose: To evaluate the capability of P947, a magnetic resonance (MR) imaging contrast agent that molecularly targets matrix metalloproteinases (MMPs), to aid detection and imaging of MMPs in atherosclerotic lesions in vivo; its specificity compared with that of P1135; expression and distribution of MMPs in atherosclerotic vessels; and in vivo distribution and molecular localization of fluorescent europium (Eu) P947.
Materials and methods: The Animal Care and Use Committee approved all experiments. P947 was synthesized by attaching a gadolinium chelate (1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid) to a peptide that specifically binds MMPs. Scrambled form of P947 (P1135) was synthesized by replacing the targeting moiety of P947 with a scrambled peptide lacking the ability to bind MMPs. P947, P1135, and gadoterate meglumine were injected into atherosclerotic apolipoprotein E-deficient and wild-type mice. The aortic MR imaging enhancement produced by the contrast agents was measured at different times and was compared by using one-way analysis of variance. MMP expression was investigated in the aortas by using MMP immunostaining and in situ MMP zymography. A fluorescent form of P947 (Eu-P947) was synthesized to compare the in vivo distribution of the contrast agent (Eu-P947) with specific MMP immunofluorescent staining.
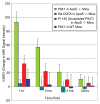
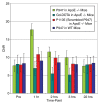
Results: MMP-targeted P947 facilitated a 93% increase (P < .001) in MR image signal intensity (contrast-to-noise ratio [CNR], 17.7 compared with 7.7; P < .001) of atherosclerotic lesions in vivo. Nontargeted P1135 (scrambled P947) provided 33% MR image enhancement (CNR, 10.8), whereas gadoterate meglumine provided 5% (CNR, 6.9). Confocal laser scanning microscopy demonstrated colocalization between fluorescent Eu-P947 and MMPs in atherosclerotic plaques. Eu-P947 was particularly present in the fibrous cap region of plaques.
Conclusion: P947 improved MR imaging for atherosclerosis through MMP-specific targeting. The results were validated and provide support for further assessment of P947 as a potential tool for the identification of unstable atherosclerosis.
Figures

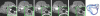
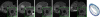







Comment in
-
Will molecular MR imaging play a role in identification and treatment of patients with vulnerable atherosclerotic plaques?Radiology. 2009 May;251(2):309-10. doi: 10.1148/radiol.2512090268. Radiology. 2009. PMID: 19401565
References
-
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque. I. Evolving concepts. J Am Coll Cardiol 2005;46:937–954. - PubMed
-
- Heart disease and stroke statistics—2006 update. American Heart Association Web site. http://www.americanheart.org/downloadable/heart/1136308648540Statupdate2.... Published 2006. Accessed September 23, 2006.
-
- Tunstall-Pedoe H. Preventing chronic diseases: a vital investment. WHO Global report. Geneva, Switzerland: World Health Organization, 2005. http://www.who.int/chp/chronic_disease_report/contents/part1.pdf. Accessed January 6, 2008.
-
- Anderson GF, Chu E. Expanding priorities: confronting chronic disease in countries with low income. N Engl J Med 2007;356:209–211. - PubMed
-
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies. II. Circulation 2003;108:1772–1778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

