The precise sequence of FGF receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations
- PMID: 19224897
- PMCID: PMC2755185
- DOI: 10.1126/scisignal.2000021
The precise sequence of FGF receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations
Abstract
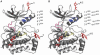
Autophosphorylation of the tyrosine kinase domain of fibroblast growth factor receptor 1 (FGFR1) is mediated by a sequential and precisely ordered three-stage autophosphorylation reaction. First-stage autophosphorylation of an activation loop tyrosine leads to 50- to 100-fold stimulation of kinase activity and is followed by second-stage phosphorylation of three additional tyrosine residues, which are binding sites for signaling molecules. Finally, third-stage phosphorylation of a second activation loop tyrosine leads to an additional 10-fold stimulation of FGFR1 catalytic activity. In this report, we show that sequential autophosphorylation of five tyrosines in the FGFR1 kinase domain is under kinetic control, mediated by both the amino acid sequence surrounding the tyrosines and their locations within the kinase structure, and, moreover, that phosphoryl transfer is the rate-limiting step. Furthermore, the strict order of autophosphorylation is disrupted by a glioblastoma-derived, oncogenic FGFR1 point mutation in the kinase domain. We propose that disrupted stepwise activation of tyrosine autophosphorylation caused by oncogenic and other activating FGFR mutations may lead to aberrant activation of and assembly of signaling molecules by the activated receptor.
Figures



References
-
- Furdui CM, Lew ED, Schlessinger J, Anderson KS. Autophosphorylation of FGFR1 kinase is mediated by a sequential and precisely ordered reaction. Mol. Cell. 2006;21:711–717. - PubMed
-
-
Abbreviations for the amino acid residues are as follows:Ala A, Cys C, Asp D, Glu E, Phe F, Gly G, His H, Ile I, Lys K, Leu L, Met M, Asn N, Pro P, Gln Q, Arg R, Ser S, Thr T, Val V, Trp W, Tyr Y. X represents any amino acid.
-
-
- Dickens M, Tavaré JM. Analysis of the order of autophosphorylation of human insulin receptor tyrosines 1158, 1162 and 1163. Biochem. Biophys. Res. Commun. 1992;186:244–250. - PubMed
-
- Wei L, Hubbard SR, Hendrickson WA, Ellis L. Expression, characterization, and crystallization of the catalytic core of the human insulin receptor protein-tyrosine kinase domain. J. Biol. Chem. 1995;270:8122–8130. - PubMed
-
- Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat. Struct. Biol. 2001;8:1058–1063. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

