Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma
- PMID: 19224914
- PMCID: PMC2667777
- DOI: 10.1074/jbc.M809551200
Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma
Abstract
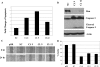
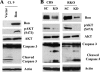
Abnormal accumulation and activation of receptor tyrosine kinase Ron (recepteur d'origine nantais) has been demonstrated in a variety of primary human cancers. We show that RNA interference-mediated knockdown of Ron kinase in a highly tumorigenic colon cancer cell line led to reduced proliferation as compared with the control cells. Decreased Ron expression sensitized HCT116 cells to growth factor deprivation stress-induced apoptosis as reflected by increased DNA fragmentation and caspase 3 activation. In addition, cell motility was decreased in Ron knockdown cells as measured by wound healing assays and transwell assays. HCT116 cells are heterozygous for gain of function mutant PIK3CA H1047R. Analysis of signaling proteins that are affected by Ron knockdown revealed that phosphatidylinositol 3-kinase (PI3K) activity of the mutant PI3K as well as AKT phosphorylation was substantially reduced in the Ron knockdown cells compared with the control cells. Moreover, we demonstrated in vivo that knockdown of Ron expression significantly reduced lung metastasis as compared with the control cells in the orthotopic models. In summary, our results demonstrate that Ron plays an essential role in maintaining malignant phenotypes of colon cancer cells through regulating mutant PI3K activity. Therefore, targeting Ron kinase could be a potential strategy for colon cancer treatment, especially in patients bearing gain of function mutant PI3K activity.
Figures

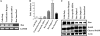

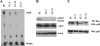

References
-
- Rubin, J. S., Bottaro, D. P., and Aaronson, S. A. (1993) Biochim. Biophys. Acta 1155 357-371 - PubMed
-
- Ronsin, C., Muscatelli, F., Mattei, M. G., and Breathnach, R. (1993) Oncogene 8 1195-1202 - PubMed
-
- Wang, M. H., Ronsin, C., Gesnel, M. C., Coupey, L., Skeel, A., Leonard, E. J., and Breathnach, R. (1994) Science 266 117-119 - PubMed
-
- Santoro, M. M., Penengo, L., Orecchia, S., Cilli, M., and Gaudino, G. (2000) Oncogene 19 5208-5211 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

