Inositol 1,4,5-triphosphate receptor-binding protein released with inositol 1,4,5-triphosphate (IRBIT) associates with components of the mRNA 3' processing machinery in a phosphorylation-dependent manner and inhibits polyadenylation
- PMID: 19224921
- PMCID: PMC2667756
- DOI: 10.1074/jbc.M807136200
Inositol 1,4,5-triphosphate receptor-binding protein released with inositol 1,4,5-triphosphate (IRBIT) associates with components of the mRNA 3' processing machinery in a phosphorylation-dependent manner and inhibits polyadenylation
Abstract
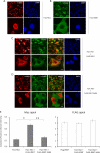
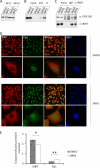
IRBIT is a recently identified protein that modulates the activities of both inositol 1,4,5-triphosphate receptor and pancreas-type Na(+)/HCO(3)(-) cotransporter 1, and the multisite phosphorylation of IRBIT is required for achieving this modulatory action. Here, we report the identification of the cleavage and polyadenylation specificity factor (CPSF), which is a multi-protein complex involved in 3' processing of mRNA precursors, as an additional binding partner for IRBIT. We found that IRBIT interacted with CPSF and was recruited to an exogenous polyadenylation signal-containing RNA. The main target for IRBIT in CPSF was Fip1 subunit, and the phosphorylation of the serine-rich region of IRBIT was required both for direct association with Fip1 in vitro and for redistribution of Fip1 into the cytoplasm of intact cells. Furthermore, tert-butylhydroquinone (tBHQ), an agent that induces oxidative stress, increased the phosphorylation level of IRBIT in vivo and in parallel enhanced the interaction between IRBIT and CPSF and promoted the cytoplasmic distribution of endogenous Fip1. In addition to CPSF, IRBIT interacted in vitro with poly(A) polymerase (PAP), which is the enzyme recruited by CPSF to elongate the poly(A) tail, and inhibited PAP activity in a phosphorylation-dependent manner. These findings raise the possibility that IRBIT modulates the polyadenylation state of specific mRNAs, both by controlling the cytoplasmic/nuclear partitioning of Fip1 and by inhibiting PAP activity, in response to a stimulus that alters its phosphorylation state.
Figures
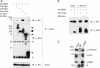




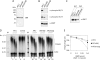
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

