Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia
- PMID: 19224993
- PMCID: PMC2668498
- DOI: 10.1128/JVI.02173-08
Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia
Abstract
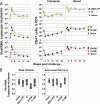
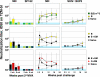
A major challenge for human immunodeficiency virus (HIV)/AIDS vaccines is the elicitation of anti-Env antibodies (Ab) capable of neutralizing the diversity of isolates in the pandemic. Here, we show that high-avidity, but nonneutralizing, Abs can have an inverse correlation with peak postchallenge viremia for a heterologous challenge. Vaccine studies were conducted in rhesus macaques using DNA priming followed by modified vaccinia Ankara boosting with HIV type 1 (HIV-1) immunogens that express virus-like particles displaying CCR5-tropic clade B (strain ADA) or clade C (IN98012) Envs. Rhesus granulocyte-macrophage colony-stimulating factor was used as an adjuvant for enhancing the avidity of anti-Env Ab responses. Challenge was with simian/human immunodeficiency virus (SHIV)-162P3, a CCR5-tropic clade B chimera of SIV and HIV-1. Within the groups receiving the clade B vaccine, a strong inverse correlation was found between the avidity of anti-Env Abs and peak postchallenge viremia. This correlation required the use of native but not gp120 or gp140 forms of Env for avidity assays. The high-avidity Ab elicited by the ADA Env had excellent breadth for the Envs of incident clade B but not clade C isolates, whereas the high-avidity Ab elicited by the IN98012 Env had excellent breadth for incident clade C but not clade B isolates. High-avidity Ab elicited by a SHIV vaccine with a dual-tropic clade B Env (89.6) had limited breadth for incident isolates. Our results suggest that certain Envs can elicit nonneutralizing but high-avidity Ab with broad potential for blunting incident infections of the same clade.
Figures

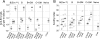


References
-
- Aasa-Chapman, M. M., S. Holuigue, K. Aubin, M. Wong, N. A. Jones, D. Cornforth, P. Pellegrino, P. Newton, I. Williams, P. Borrow, and A. McKnight. 2005. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 792823-2830. - PMC - PubMed
-
- Ahmad, A., and J. Menezes. 1996. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 10258-266. - PubMed
-
- Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4107-112. - PubMed
-
- Blumberg, R. S., T. Paradis, K. L. Hartshorn, M. Vogt, D. D. Ho, M. S. Hirsch, J. Leban, V. L. Sato, and R. T. Schooley. 1987. Antibody-dependent cell-mediated cytotoxicity against cells infected with the human immunodeficiency virus. J. Infect. Dis. 156878-884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

