Role of defective Oct-2 and OCA-B expression in immunoglobulin production and Kaposi's sarcoma-associated herpesvirus lytic reactivation in primary effusion lymphoma
- PMID: 19224997
- PMCID: PMC2668464
- DOI: 10.1128/JVI.02196-08
Role of defective Oct-2 and OCA-B expression in immunoglobulin production and Kaposi's sarcoma-associated herpesvirus lytic reactivation in primary effusion lymphoma
Abstract
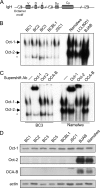
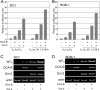
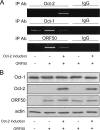
Primary effusion lymphoma (PEL) is a distinct type of B-cell non-Hodgkin lymphoma characterized by the presence of Kaposi's sarcoma-associated herpesvirus (KSHV/human herpesvirus 8). Despite having a genotype and gene expression signature of highly differentiated B cells, PEL does not usually express surface or cytoplasmic immunoglobulin (Ig). We show the lack of Oct-2 and OCA-B transcription factors to be responsible, at least in part, for this defect in Ig production. Like Ig genes, ORF50, the key regulator of the switch from latency to lytic reactivation, contains an octamer motif within its promoter. We therefore examined the impact of Oct-2 and OCA-B on ORF50 activation. The binding of Oct-1 to the ORF50 promoter has been shown to significantly enhance ORF50 transactivation. We found that Oct-2, on the other hand, inhibited ORF50 expression and consequently lytic reactivation by competing with Oct-1 for the octamer motif in the ORF50 promoter. Our data suggest that Oct-2 downregulation in infected cells would be favorable to KSHV in allowing for efficient viral reactivation.
Figures




References
-
- Annweiler, A., S. Zwilling, R. A. Hipskind, and T. Wirth. 1993. Analysis of transcriptional stimulation by recombinant Oct proteins in a cell-free system. J. Biol. Chem. 2682525-2534. - PubMed
-
- Ansari, M. Q., D. B. Dawson, R. Nador, C. Rutherford, N. R. Schneider, M. J. Latimer, L. Picker, D. M. Knowles, and R. W. McKenna. 1996. Primary body cavity-based AIDS-related lymphomas. Am. J. Clin. Pathol. 105221-229. - PubMed
-
- Arguello, M., M. Sgarbanti, E. Hernandez, Y. Mamane, S. Sharma, M. Servant, R. Lin, and J. Hiscott. 2003. Disruption of the B-cell specific transcriptional program in HHV-8 associated primary effusion lymphoma cell lines. Oncogene 22964-973. - PubMed
-
- Banerji, J., L. Olson, and W. Schaffner. 1983. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell 33729-740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

