K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks
- PMID: 19225109
- PMCID: PMC2644652
- DOI: 10.1073/pnas.0811277106
K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks
Abstract
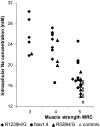
Normal resting potential (P1) of myofibers follows the Nernst equation, exhibiting about -85 mV at a normal extracellular K(+) concentration ([K(+)](o)) of 4 mM. Hyperpolarization occurs with decreased [K(+)](o), although at [K(+)](o) < 1.0 mM, myofibers paradoxically depolarize to a second stable potential of -60 mV (P2). In rat myofiber bundles, P2 also was found at more physiological [K(+)](o) and was associated with inexcitability. To increase the relative frequency of P2 to 50%, [K(+)](o) needed to be lowered to 1.5 mM. In the presence of the ionophore gramicidin, [K(+)](o) reduction to only 2.5 mM yielded the same effect. Acetazolamide normalized this increased frequency of P2 fibers. The findings mimic hypokalemic periodic paralysis (HypoPP), a channelopathy characterized by hypokalemia-induced weakness. Of myofibers from 7 HypoPP patients, up to 25% were in P2 at a [K(+)](o) of 4 mM, in accordance with their permanent weakness, and up to 99% were in P2 at a [K(+)](o) of 1.5 mM, in accordance with their paralytic attacks. Of 36 HypoPP patients, 25 had permanent weakness and myoplasmic intracellular Na(+) ([Na(+)](i)) overload (up to 24 mM) as shown by in vivo (23)Na-MRI. Acetazolamide normalized [Na(+)](i) and increased muscle strength. HypoPP myofibers showed a nonselective cation leak of 12-19.5 microS/cm(2), which may explain the Na(+) overload. The leak sensitizes myofibers to reduced serum K(+), and the resulting membrane depolarization causes the weakness. We postulate that the principle of paradoxical depolarization and loss of function upon [K(+)](o) reduction may apply to other tissues, such as heart or brain, when they become leaky (e.g., because of ischemia).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Venance SL, et al. The primary periodic paralyses: Diagnosis, pathogenesis and treatment. Brain. 2006;129:8–17. - PubMed
-
- Links TP, Zwarts MJ, Wilmink JT, Molenaar WM, Oosterhuis HJ. Permanent muscle weakness in familial hypokalaemic periodic paralysis. Clinical, radiological and pathological aspects. Brain. 1990;113:1873–1889. - PubMed
-
- Rüdel R, Lehmann-Horn F, Ricker K, Küther G. Hypokalemic periodic paralysis: In vitro investigation of muscle fiber membrane parameters. Muscle Nerve. 1984;7:110–120. - PubMed
-
- Ruff RL. Insulin acts in hypokalemic periodic paralysis by reducing inward rectifier K+ current. Neurology. 1999;53:1556–1563. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

