An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation
- PMID: 19225150
- PMCID: PMC2663935
- DOI: 10.1091/mbc.e08-12-1250
An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation
Abstract
The TOR kinases are conserved negative regulators of autophagy in response to nutrient conditions, but the signaling mechanisms are poorly understood. Here we describe a complex containing the protein kinase Atg1 and the phosphoprotein Atg13 that functions as a critical component of this regulation in Drosophila. We show that knockout of Atg1 or Atg13 results in a similar, selective defect in autophagy in response to TOR inactivation. Atg1 physically interacts with TOR and Atg13 in vivo, and both Atg1 and Atg13 are phosphorylated in a nutrient-, TOR- and Atg1 kinase-dependent manner. In contrast to yeast, phosphorylation of Atg13 is greatest under autophagic conditions and does not preclude Atg1-Atg13 association. Atg13 stimulates both the autophagic activity of Atg1 and its inhibition of cell growth and TOR signaling, in part by disrupting the normal trafficking of TOR. In contrast to the effects of normal Atg13 levels, increased expression of Atg13 inhibits autophagosome expansion and recruitment of Atg8/LC3, potentially by decreasing the stability of Atg1 and facilitating its inhibitory phosphorylation by TOR. Atg1-Atg13 complexes thus function at multiple levels to mediate and adjust nutrient-dependent autophagic signaling.
Figures

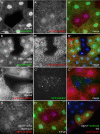

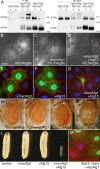
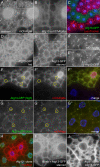
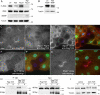
References
-
- Chan E. Y., Kir S., Tooze S. A. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 2007;282:25464–25474. - PubMed
-
- Funakoshi T., Matsuura A., Noda T., Ohsumi Y. Analyses of APG13 gene involved in autophagy in yeast, Saccharomyces cerevisiae. Gene. 1997;192:207–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

