Regulation of distinct septin rings in a single cell by Elm1p and Gin4p kinases
- PMID: 19225152
- PMCID: PMC2669037
- DOI: 10.1091/mbc.e08-12-1169
Regulation of distinct septin rings in a single cell by Elm1p and Gin4p kinases
Abstract
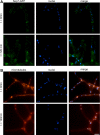
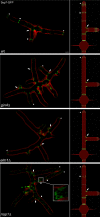
Septins are conserved, GTP-binding proteins that assemble into higher order structures, including filaments and rings with varied cellular functions. Using four-dimensional quantitative fluorescence microscopy of Ashbya gossypii fungal cells, we show that septins can assemble into morphologically distinct classes of rings that vary in dimensions, intensities, and positions within a single cell. Notably, these different classes coexist and persist for extended times, similar in appearance and behavior to septins in mammalian neurons and cultured cells. We demonstrate that new septin proteins can add through time to assembled rings, indicating that septins may continue to polymerize during ring maturation. Different classes of rings do not arise from the presence or absence of specific septin subunits and ring maintenance does not require the actin and microtubule cytoskeletons. Instead, morphological and behavioral differences in the rings require the Elm1p and Gin4p kinases. This work demonstrates that distinct higher order septin structures form within one cell because of the action of specific kinases.
Figures







References
-
- Alberti-Segui C., Dietrich F., Altmann-Johl R., Hoepfner D., Philippsen P. Cytoplasmic dynein is required to oppose the force that moves nuclei towards the hyphal tip in the filamentous ascomycete Ashbya gossypii. J. Cell Sci. 2001;114:975–986. - PubMed
-
- Ayad-Durieux Y., Knechtle P., Goff S., Dietrich F., Philippsen P. A PAK-like protein kinase is required for maturation of young hyphae and septation in the filamentous ascomycete Ashbya gossypii. J. Cell Sci. 2000;113:4563–4575. - PubMed
-
- Barral Y., Mermall V., Mooseker M. S., Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell. 2000;5:841–851. - PubMed
-
- Bertin A., McMurray M. A., Grob P., Park S. S., Garcia G., 3rd, Patanwala I., Ng H. L., Alber T., Thorner J., Nogales E. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc. Natl. Acad. Sci. USA. 2008;105:8274–8279. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

