Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology
- PMID: 19225153
- PMCID: PMC2663937
- DOI: 10.1091/mbc.e08-11-1126
Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology
Abstract
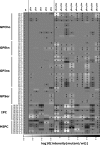
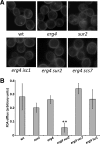
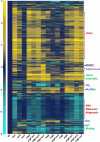
Sterols and sphingolipids are limited to eukaryotic cells, and their interaction has been proposed to favor formation of lipid microdomains. Although there is abundant biophysical evidence demonstrating their interaction in simple systems, convincing evidence is lacking to show that they function together in cells. Using lipid analysis by mass spectrometry and a genetic approach on mutants in sterol metabolism, we show that cells adjust their membrane composition in response to mutant sterol structures preferentially by changing their sphingolipid composition. Systematic combination of mutations in sterol biosynthesis with mutants in sphingolipid hydroxylation and head group turnover give a large number of synthetic and suppression phenotypes. Our unbiased approach provides compelling evidence that sterols and sphingolipids function together in cells. We were not able to correlate any cellular phenotype we measured with plasma membrane fluidity as measured using fluorescence anisotropy. This questions whether the increase in liquid order phases that can be induced by sterol-sphingolipid interactions plays an important role in cells. Our data revealing that cells have a mechanism to sense the quality of their membrane sterol composition has led us to suggest that proteins might recognize sterol-sphingolipid complexes and to hypothesize the coevolution of sterols and sphingolipids.
Figures



Comment in
-
An MBoC favorite: Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology.Mol Biol Cell. 2012 Jul;23(13):2402. doi: 10.1091/mbc.E12-03-0190. Mol Biol Cell. 2012. PMID: 22745339 Free PMC article. No abstract available.
References
-
- Ahmed S. N., Brown D. A., London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. - PubMed
-
- Aittoniemi J., Rog T., Niemela P., Pasenkiewicz-Gierula M., Karttunen M., Vattulainen I. Tilt: major factor in sterols' ordering capability in membranes. J. Phys. Chem. B Condens. Matter Mater. Surf. Interfaces Biophys. 2006;110:25562–25564. - PubMed
-
- Anderson R. G., Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

