Cyclin D1 as a universally expressed mantle cell lymphoma-associated tumor antigen for immunotherapy
- PMID: 19225534
- PMCID: PMC5890427
- DOI: 10.1038/leu.2009.19
Cyclin D1 as a universally expressed mantle cell lymphoma-associated tumor antigen for immunotherapy
Abstract
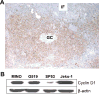
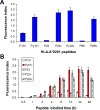
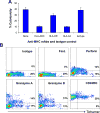
Mantle cell lymphoma (MCL) accounts for 5-10% of all non-Hodgkin lymphomas and has the worst prognosis among all lymphomas. The hallmark of MCL is a t(11;14) translocation that results in overexpression of cyclin D1 by tumor cells of virtually all patients. In this study, we examined whether cyclin D1 could be an effective tumor-associated antigen for immunotherapy. We identified cyclin D1 peptides for HLA-A(*)0201 and generated peptide-specific CD8(+) T-cell lines from HLA-A(*)0201(+) blood donors and MCL patients. These cell lines proliferated in response to cyclin D1 peptide-pulsed stimulatory cells. Moreover, the T cells efficiently lysed peptide-pulsed but not unpulsed T2 cells and autologous dendritic cells; cyclin D1(+) and HLA-A(*)0201(+) human MCL lines MINO, SP53, Jeko-1 and Granta 519; and more importantly, HLA-A(*)0201(+) primary lymphoma cells from MCL patients. No killing was observed with HLA-A(*)0201(-) primary lymphoma cells or HLA-A(*)0201(+) normal blood cells, including B cells. These results indicate that these T cells are potent cytotoxic T cells and recognize cyclin D1 peptides naturally presented by patient lymphoma cells in the context of HLA-A(*)0201 molecules. Taken together, our work identifies cyclin D1 as a potentially important antigen for immunotherapy of MCL.
Figures


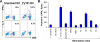
Comment in
-
Identification of native, immunogenic peptides from Cyclin D1.Leukemia. 2010 Jan;24(1):209-11. doi: 10.1038/leu.2009.184. Epub 2009 Sep 10. Leukemia. 2010. PMID: 19741723 No abstract available.
References
-
- Pinyol M, Bea S, Pla L, Ribrag V, Bosq J, Rosenwald A, et al. Inactivation of RB1 in mantle-cell lymphoma detected by nonsense-mediated mRNA decay pathway inhibition and microarray analysis. Blood. 2007;109:5422–5429. - PubMed
-
- Zelenetz AD. Mantle cell lymphoma: an update on management. Ann Oncol. 2006;17(Suppl 4):iv12–14. - PubMed
-
- Martin P, Leonard JP. Novel therapeutic targets in mantle cell lymphoma. Expert Opin Ther Targets. 2007;11:929–940. - PubMed
-
- Evens AM, Winter JN, Hou N, Nelson BP, Rademaker A, Patton D, et al. A phase II clinical trial of intensive chemotherapy followed by consolidative stem cell transplant: long-term follow-up in newly diagnosed mantle cell lymphoma. Br J Haematol. 2008;140:385–393. - PubMed
-
- Bertoni F, Ponzoni M. The cellular origin of mantle cell lymphoma. Int J Biochem Cell Biol. 2007;39:1747–1753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

