Many specialists for suppressing cortical excitation
- PMID: 19225588
- PMCID: PMC2622740
- DOI: 10.3389/neuro.01.026.2008
Many specialists for suppressing cortical excitation
Abstract
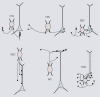
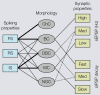
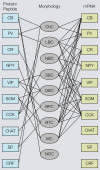
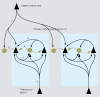
Cortical computations are critically dependent on GABA-releasing neurons for dynamically balancing excitation with inhibition that is proportional to the overall level of activity. Although it is widely accepted that there are multiple types of interneurons, defining their identities based on qualitative descriptions of morphological, molecular and physiological features has failed to produce a universally accepted 'parts list', which is needed to understand the roles that interneurons play in cortical processing. A list of features has been published by the Petilla Interneurons Nomenclature Group, which represents an important step toward an unbiased classification of interneurons. To this end some essential features have recently been studied quantitatively and their association was examined using multidimensional cluster analyses. These studies revealed at least 3 distinct electrophysiological, 6 morphological and 15 molecular phenotypes. This is a conservative estimate of the number of interneuron types, which almost certainly will be revised as more quantitative studies will be performed and similarities will be defined objectively. It is clear that interneurons are organized with physiological attributes representing the most general, molecular characteristics the most detailed and morphological features occupying the middle ground. By themselves, none of these features are sufficient to define classes of interneurons. The challenge will be to determine which features belong together and how cell type-specific feature combinations are genetically specified.
Keywords: GABAergic neurons; cerebral cortex; inhibition; interneurons.
Figures
References
-
- Angulo M. C., Rossier J., Staiger J. F., Audinat E. (1999). Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J. Neurophysiol. 82, 1295–1302 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

