New age of neuroproteomics in Alzheimer's disease research
- PMID: 19225878
- PMCID: PMC11506148
- DOI: 10.1007/s10571-009-9358-6
New age of neuroproteomics in Alzheimer's disease research
Abstract
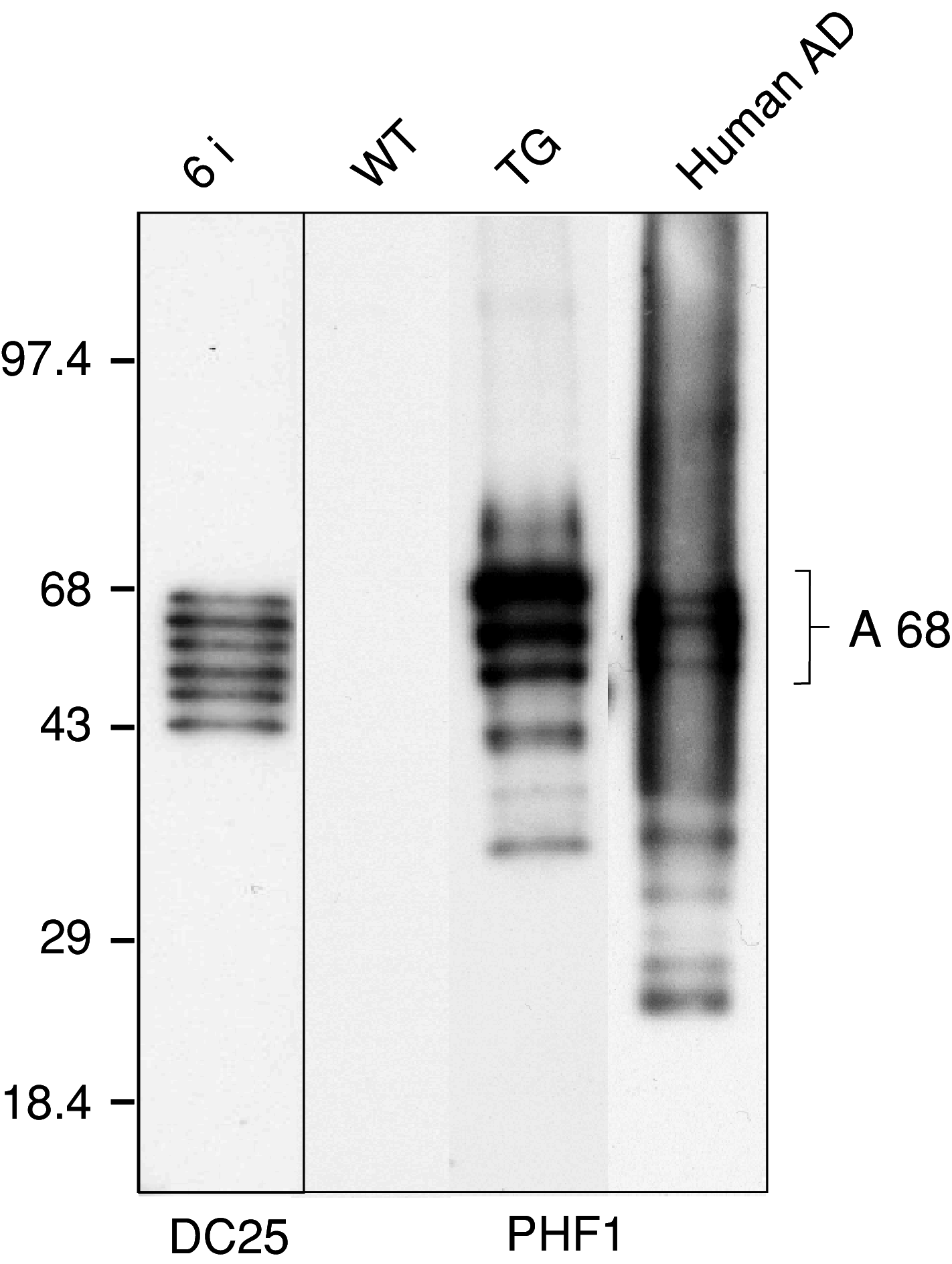
Alzheimer's disease (AD) is the leading cause of dementia, a condition that gradually destroys brain cells and leads to progressive decline in mental functions. The disease is characterized by accumulation of misfolded neuronal proteins, amyloid and tau, into insoluble aggregates known as extracellular senile plaques and intracellular neurofibrillary tangles, respectively. However, only tau pathology appears to correlate with the progression of the disease and it is believed to play a central role in the progression of neurodegeneration. In AD, tau protein undergoes various types of posttranslational modifications, most notably hyperphosphorylation and truncation. Using four proteomics approaches we aimed to uncover the key steps leading to neurofibrillary degeneration and thus to identify therapeutic targets for AD. Functional neuroproteomics was employed to generate the first transgenic rat model of AD by expressing a truncated misordered form of tau, "Alzheimer's tau". The rat model showed that Alzheimer's tau toxic gain of function is responsible for the induction of abnormal tau cascade and is the driving force in the development of neurofibrillary degeneration. Structural neuroproteomics allowed us to determine partial 3D structure of the Alzheimer's filament core at a resolution of 1.6 A. Signaling neuroproteomics data lead to the identification and characterization of relevant phosphosites (the tau phosphosignalome) contributing to neurodegeneration. Interaction neuroproteomics revealed links to a new group of proteins interacting with Alzheimer's tau (tau interactome) under normal and pathological conditions, which would provide novel drug targets and novel biomarkers for treatment of AD and other tauopathies.
Figures

Similar articles
-
Tau truncation is a productive posttranslational modification of neurofibrillary degeneration in Alzheimer's disease.Curr Alzheimer Res. 2010 Dec;7(8):708-16. doi: 10.2174/156720510793611556. Curr Alzheimer Res. 2010. PMID: 20678071 Review.
-
Phosphorylated tau interactome in the human Alzheimer's disease brain.Brain. 2020 Sep 1;143(9):2803-2817. doi: 10.1093/brain/awaa223. Brain. 2020. PMID: 32812023 Free PMC article.
-
Transition of tau protein from disordered to misordered in Alzheimer's disease.Neurodegener Dis. 2010;7(1-3):24-7. doi: 10.1159/000283478. Epub 2010 Feb 13. Neurodegener Dis. 2010. PMID: 20160453
-
Pathological Changes of Tau Related to Alzheimer's Disease.ACS Chem Neurosci. 2019 Feb 20;10(2):931-944. doi: 10.1021/acschemneuro.8b00457. Epub 2018 Oct 23. ACS Chem Neurosci. 2019. PMID: 30346708 Review.
-
Alpha1-antichymotrypsin, an inflammatory protein overexpressed in Alzheimer's disease brain, induces tau phosphorylation in neurons.Brain. 2006 Nov;129(Pt 11):3020-34. doi: 10.1093/brain/awl255. Epub 2006 Sep 20. Brain. 2006. PMID: 16987932
Cited by
-
Causes versus effects: the increasing complexities of Alzheimer's disease pathogenesis.Expert Rev Neurother. 2010 May;10(5):683-91. doi: 10.1586/ern.10.27. Expert Rev Neurother. 2010. PMID: 20420489 Free PMC article. Review.
-
Genomic and proteomic responses to environmentally relevant exposures to dieldrin: indicators of neurodegeneration?Toxicol Sci. 2010 Sep;117(1):190-9. doi: 10.1093/toxsci/kfq192. Epub 2010 Jun 27. Toxicol Sci. 2010. PMID: 20584760 Free PMC article.
-
Quantitative proteomics analysis of phosphorylated proteins in the hippocampus of Alzheimer's disease subjects.J Proteomics. 2011 Jun 10;74(7):1091-103. doi: 10.1016/j.jprot.2011.03.033. Epub 2011 Apr 13. J Proteomics. 2011. PMID: 21515431 Free PMC article.
-
Exploring temporal and sex-linked dysregulation in Alzheimer disease phosphoproteome.iScience. 2024 Sep 13;27(10):110941. doi: 10.1016/j.isci.2024.110941. eCollection 2024 Oct 18. iScience. 2024. PMID: 39391719 Free PMC article.
-
Tau Protein and Its Role in Blood-Brain Barrier Dysfunction.Front Mol Neurosci. 2020 Sep 30;13:570045. doi: 10.3389/fnmol.2020.570045. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33100967 Free PMC article. Review.
References
-
- Aebersold R, Mann M (2003) Mass spectrometry-based proteomics. Nature 422(6928):198–207. doi:10.1038/nature01511 - PubMed
-
- Alonso AC, Grundke-Iqbal I, Iqbal K (1996) Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 2(7):783–787. doi:10.1038/nm0796-783 - PubMed
-
- Alzheimer A (1907) Über eine eigenartigeErkrankung der Hirnrinde. Allg Z Psychiatry Psych-Gerichtl Med 64:146–148
-
- Baker M, Kwok JB, Kucera S, Crook R, Farrer M, Houlden H, Isaacs A, Lincoln S, Onstead L, Hardy J, Wittenberg L, Dodd P, Webb S, Hayward N, Tannenberg T, Andreadis A, Hallupp M, Schofield P, Dark F, Hutton M (1997) Localization of frontotemporal dementia with parkinsonism in an Australian kindred to chromosome 17q21–22. Ann Neurol 42(5):794–798. doi:10.1002/ana.410420516 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

