Intramembrane proteolysis
- PMID: 19226105
- PMCID: PMC2667872
- DOI: 10.1021/cr8004197
Intramembrane proteolysis
Figures
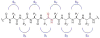
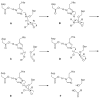

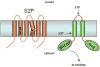
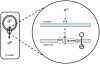


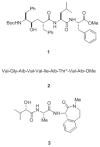
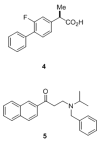

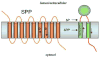
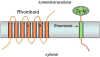
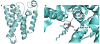
References
-
- Barrett AJ, Rawlings ND, Woessner JF, editors. The Handbook of Proteolytic Enzymes. Academic Press, Ltd.; Amsterdam: 2004.
-
- Turk B. Nat Rev Drug Discov. 2006;5:785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

