Glycogen contributes to the environmental persistence and transmission of Vibrio cholerae
- PMID: 19226328
- PMCID: PMC2704980
- DOI: 10.1111/j.1365-2958.2009.06629.x
Glycogen contributes to the environmental persistence and transmission of Vibrio cholerae
Abstract
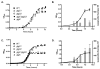
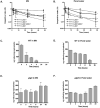
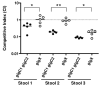
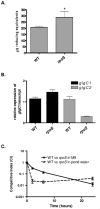
Pathogenic Vibrio cholerae cycle between the nutrient-rich human intestinal tract and nutrient-poor aquatic environments and currently few bacterial factors are known that aid in the transition between these disparate environments. We hypothesized that the ability to store carbon as glycogen would facilitate both bacterial fitness in the aquatic environment and transmission of V. cholerae to new hosts. To investigate the role of glycogen in V. cholerae transmission, we constructed mutants that cannot store or degrade glycogen. Here, we provide the first report of glycogen metabolism in V. cholerae and demonstrate that glycogen prolongs survival in nutrient-poor environments that are known ecological niches of V. cholerae, including pond water and rice-water stool. Additionally, glycogen contributes to the pathogenesis of V. cholerae in a transmission model of cholera. A role for glycogen in the transmission of V. cholerae is further supported by the presence of glycogen granules in rice-water stool vibrios from cholera patients, indicating that glycogen is stored during human infection. Collectively, our findings indicate that glycogen metabolism is critical for V. cholerae to transition between host and aquatic environments.
Figures

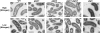
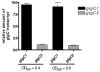

References
-
- Alonso-Casajus N, Dauvillee D, Viale AM, Munoz FJ, Baroja-Fernandez E, Moran-Zorzano MT, Eydallin G, Ball S, Pozueta-Romero J. Glycogen phosphorylase, the product of the glgP Gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Escherichia coli. J Bacteriol. 2006;188:5266–5272. - PMC - PubMed
-
- Andersson RA, Koiv V, Norman-Setterblad C, Pirhonen M. Role of RpoS in virulence and stress tolerance of the plant pathogen Erwinia carotovora subsp. carotovora. Microbiology. 1999;145(Pt 12):3547–3556. - PubMed
-
- Bonafonte MA, Solano C, Sesma B, Alvarez M, Montuenga L, Garcia-Ros D, Gamazo C. The relationship between glycogen synthesis, biofilm formation and virulence in salmonella enteritidis. FEMS Microbiol Lett. 2000;191:31–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

